
EDUARDO CESARUSP researchers are making artificial skin with the goal of developing a commercial kit for testing cosmeticsEDUARDO CESAR
In Brazil, a link between public laboratories, research groups and government organizations to curtail or eliminate the use of animals for testing product safety and effectiveness is gaining strength. The effort exploded in 2012 when the federal government established the National Network for Alternative Methods (Renama) and when the National Council for Scientific and Technological Development (CNPq) issued a request for proposals for 10 Renama research projects. All of the projects are under way and they focus on different things, such as producing artificial skin kits to test cosmetics for sensitivity, studies of larvae capable of replacing mammals in toxicity tests or reducing the number of rodents used to control vaccine quality. Three laboratories are part of the central core at Renama. One of them is the Brazilian Biosciences National Laboratory (LNBio) in Campinas. The others are in Rio de Janeiro: the National Institute for Quality Control in Health (INCQS), part of the Oswaldo Cruz Foundation; and the National Institute of Metrology, Quality, and Technology (Inmetro).
In March, this organization, still in the consolidation phase, was given the challenge of meeting an ambitious goal: provide support to phase out the use test animals over the next five years, provided a validated alternative exists. For new methods that have yet to be validated, this process will involve the Brazilian Center for the Validation of Alternative Methods (BraCVAM) and all of Renama. The National Council for the Control of Animal Experimentation (Concea), a collegial institution that is part of the Ministry of Science, Technology and Innovation (MCTI), made the decision to replace test animals. Since 2009 it has been in charge of developing animal experimentation standards in Brazil and replacing animals for scientific and educational proposals whenever alternative resources exist. In May, BraCVAM made its first recommendation to Concea on alternative methods that had already been validated and that are internationally accepted. There are 17 techniques that deal with skin sensitivity, the potential for eye irritation and deterioration and toxicity. “With this resolution, Brazil will be able to actually adopt alternative methods for testing pesticides, cosmetics and drugs,” says the coordinator of Concea, José Mauro Granjeiro.
The greatest potential for replacing animals with alternative methods is not in scientific research in the academic arena, but in tests that the regulatory agencies require to ensure that products are safe and effective. “Experiments with animals that have been conducted to prove scientific hypotheses are designed independently by researchers. Each one has a different question and designs a specific set of experiments to answer the question. Therefore, standardizing the experiments is much more difficult,” explains Eduardo Pagani, researcher and manager of development of pharmaceuticals at the LNBio. “The tests that agencies everywhere in the world require for cosmetics and other products are always performed in accordance with standardized methods. In these tests there is more room for proposing alternatives that do not use animals,” he observes. The requirement to perform in vivo testing to register drugs and cosmetics began in the 1960s after the well-known accident with thalidomide. The drug was sold all over the world as a treatment to control nausea during pregnancy. Thousands of mothers that used the drug gave birth to deformed children. The movement for replacing animal models with alternative methods ramped up in 2003 when Europe proposed banning the use of animals in cosmetic testing, and then it took two decades to implement it.

EDUARDO CESARProduction of artificial skin by the group of USP professor Silvya Stuchi-Maria EnglerEDUARDO CESAR
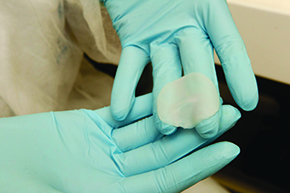
The projects for alternative methods that the MCTI supported in 2012 were divided into two segments. In one segment, the goal was to identify groups that were already working with alternative methods and to support studies they performed. Nine projects of groups in the states of São Paulo, Bahia, Goiás, Rio de Janeiro, Santa Catarina and Rio Grande do Sul were selected. The second segment had a specific focus: develop the capability in Brazil to mass produce artificial skin kits used by the cosmetic industry to test the safety of their imported products that became problematic in Brazil. It turns out that the kits with live cells became unusable in just a few days and long waits in customs often made it impractical to purchase them. As a result, companies performed these tests abroad.
The project that was considered came from a group at the USP School of Pharmaceutical Sciences, led by Silvya Stuchi-Maria Engler, who began producing artificial skin around 2005 with FAPESP support (see Pesquisa FAPESP Issue No. 166). Produced from cells taken from donors, the skin reproduces the same human biological tissue and can be used to assess the toxicity and effectiveness of new compounds for pharmaceuticals and cosmetics. At first, the purpose of research on artificial skin was to support the other line of investigation in which Stuchi is involved: the study of molecules capable of controlling melanoma, a very aggressive skin tumor. “We quickly saw that the skin could be useful to businesses,” she says. “The kits are an alternative for testing cosmetics, but it is good to point out that the use of animals is still vital, for example, in tests for developing drugs,” Stuchi observes.
The Butantan Institute, whose role is to develop and produce serums and vaccines, is cutting down on the number of animals such as mice and guinea pigs that are used for quality control. This effort has already brought about progress in many areas, including a more than 60% reduction in the use of mice for testing the quality of the Hepatitis B recombinant vaccine by developing an immunosorbent test with equivalent functions. Through its work, the Institute was able to submit a project for the Renama request for proposals that involved many alternative methods to control the quality of vaccines and serums. In one of the areas of research, the goal is to lower the number of animals in the tests in lots of diphtheria and tetanus vaccines by using in vitro testing to detect immunogenic activity. In another, the goal is to replace testing using guinea pigs with trials in cells to control the diphtheria anatoxin, a diphtheria toxin that maintains immunogenic activity, although it is no longer toxic. A third focus is to adapt a kit to vaccines that the Institute produces to replace the use of rabbits in tests for pyrogens, contaminants that cause fever and that can be derived from microorganisms or protein agglomerations. The fourth area is to attempt to lessen the use of mice in serology for the whooping cough vaccine; the idea is to use for the disease the same animals that are used to dispense antibodies that fight diphtheria and tetanus. The decrease in the number is significant: down from 170 animals per lot of vaccine to just six guinea pigs.
Finally, Butantan has already seen success with a technique that has the potential to replace the use of mice with immunoenzymatic testing in one stage of production of the rabies vaccine. “There’s no turning back once you cut down on the number and use of animals,” says chemist Wagner Quintilio, the researcher at Butantan who is in charge of the project. “There is the pressure from society and research ethics committees that will not accept excessive animal use. There is economic pressure as well. It is expensive to raise animals in adequate conditions and they take up a lot of space,” he says.
EDUARDO CESARArtificial skinEDUARDO CESAR
The project of the group led by mycologist Maria José Giannini, professor at the Araraquara School of Pharmaceutical Sciences at the São Paulo State University (Unesp), plans to establish the Center for the Development and Validation of Alternative Methods (Cedevam) to develop and test techniques that curb the use of animals. Giannini is the supervisor for post-doctorate student Liliana Scorzoni, who is in charge of research on models that can replace mammals with other organisms in microbe virulence and drug effectiveness tests. The most advanced front is the Galleria mellonella, a species of lepidopteron insect whose larva is useful for verifying the activity of certain substances. “It is easy to handle and can minimize the use of animals,” says Giannini, who is also a member of the FAPESP Board of Trustees. “The larva has cells similar to the cells in the immune system. When a toxic substance is injected into it, it reacts and darkens,” she says. It is expected that the Galleria will replace other animals such as rats and mice for at least some stage of toxicity and virulence testing.
Another alternative model that the Unsesp group is eying is the C. elegans, a nematode that is one millimeter long and is susceptible to infection from pathogenic bacteria and fungi. “It has an immune system for recognizing and eliminating pathogens that is very similar to the system in vertebrae. In addition, its genome has been completely sequenced, while the genome of the Galleria has not,” Giannini says. Both models are being tested to evaluate the virulence of Paracoccidioides fungi, which are endemic in Latin America. Other models such as zebrafish will be tested. In 2010, the Office of the Dean for Research at Unesp, which Giannini heads, organized an international forum in São Paulo to discuss alternatives to using animals for toxicity testing. The forum brought together authorities such as Thomas Hartung, Director of the Center for Alternatives to Animal Testing at Johns Hopkins University. “The search for alternative models is also important for developing more efficient methods. Animal models have limitations and sometimes their guarantees of safety are insufficient, as illustrated by drugs that are approved but are eventually withdrawn from the market,” Giannini says.
The decision by Concea to push for the recognition of validated alternative methods was a response to a petition from Humane Society International, a nongovernmental organization that demanded banning animal testing for cosmetics. In São Paulo State, the use of animals for testing cosmetics is banned by a state law that was enacted in January 2014. Concea, which did not accept the petition, realized that accelerating the implementation of alternative techniques would be more effective in limiting the use of animals for testing cosmetics than just an exclusive ban on such use, since animals are practically no longer used for this purpose. “A total ban would place public safety at risk,” says physician and biophysicist Marcelo Morales, professor at the Federal University of Rio de Janeiro (UFRJ) and former coordinator of Concea. “A total ban could make it impossible to develop cosmetics with new ingredients or molecules discovered in our biodiversity that contain unknown contaminants,” he says. Luiz Henrique do Canto Pereira, general coordinator of biotechnology and health at the MCTI, states that the ban could interfere with the MCTI strategy of replacing, reducing and improving the use of animals in tests whenever possible. “The campaign for the ban is destroying the effort we have been making since 2011, when we began to design this program to organize a structured network in Brazil that would be capable of validating and more broadly disseminating alternative methods, and not just for cosmetics, but for pharmaceuticals and pesticides as well,” he says. “Even in Europe there are safeguards that allow tests to be performed if the health of the public is at risk.”

EDUARDO CESARZebrafish, whose larvae can replace animals in toxicity tests: alternative modelsEDUARDO CESAR
Some are in favor of shortening the five-year deadline for the replacement that Concea recommends. “Recently we have begun to invest in developing alternative methods here in Brazil and now we run the risk of snatching defeat from the jaws of victory if we fail to achieve results immediately,” says Maria José Giannini of Unesp. “Because they are under deadline pressure, companies may import technicians instead of using home-grown expertise. This is already happening today. Cosmetics companies report that they do not conduct tests with animals in Brazil; instead, they do so in other countries to make sure that the products are safe,” she explains.
Octavio Presgrave, coordinator of BraCVAM, expects that internationally accepted practices will be approved soon. “For domestic validation, it will have to be demonstrated that the data already obtained abroad is reproduced in the tests we conduct in our laboratories,” says Presgrave, a researcher at the National Institute for Quality Control in Health (INCQS). According to Presgrave, the five-year deadline is feasible. “It is enough time for the companies and laboratories to adjust,” he says. In other cases, BraCVAM’s work will take more time. This is true, for example, of the Het-Cam protocol, which aims to replace the use of rabbits with a membrane from the eggs of hens to identify compounds that cause deterioration or significant irritation. The method, established in Europe in 1985, is accepted only as a pre-test in France and Germany. The Hat-Cam process will be the first validation study in Brazil that follows international principles, Presgrave says. “When we stop using animals in a test, the gain in ethics is major. But a new method also means creating knowledge. We are developing innovations to find methods that are more reliable and sensitive,” he says.
In another area to curb the use of animals in laboratory testing, the LNBio is receiving funding from the MCTI to create a center for in silico testing in order to use fewer animals in research for drugs. In silico refers to the silicon used in integrated circuits and means “in computers.” This term was coined as an analogy to the terms in vivo and in vitro, which have been in use for a long time. In silico testing involves computer simulations to determine, for example, whether molecules that are being considered for use in new drugs can really be used for this purpose. “Computers are able to compare the structure of the candidate molecule with the structure of others that have already been tested and whose characteristics are stored in databases to determine whether it is worthwhile to continue to develop them,” says Eduardo Pagani from the LNBio. These tests can also assist in determining whether a given molecule, even one with potential, has a real chance of being absorbed by the organism if it is administered orally. According to recognized estimates, of the five to ten thousand molecules that are evaluated initially for potential activity in a target, 250 are synthesized and move on to testing in animals, while five move on to clinical testing in humans, and just one reaches the market as a drug. “The purpose of in silico testing is to further decrease the number of substances that are tested using animals in order to quickly eliminate those that are shown to be not feasible. It is a screening process we use so that we do not waste time or financial resources, and most of all it prevents the unjustifiable use of animals in projects that are predictably doomed to fail.”

EDUARDO CESARCell cultures for testing cytotoxicity for a diphtheria anatoxin to replace the use of guinea pigs to control the quality of the diphtheria vaccineEDUARDO CESAR
Last month the LNBio released the results of a request for proposals that gave companies, research institutes and universities the opportunity to conduct in silico tests in the laboratory. Seven companies submitted 19 proposals. “All were accepted and we will begin the testing in the coming months,” says Tiago Sobreira, a bioinformatics researcher at the LNBio in charge of the operational part of the in silico tests. The companies expressed interest in submitting proposals and now they will negotiate the terms of their involvement, and this will include protecting industrial confidentiality. Some of the labs being considered are Farmanguinhos, Cristália and Eurofarma, as well as cosmetics companies such as Boticário and Natura. “Anyone who develops pharmaceuticals says that it will take 15 years and cost R$1 billion to bring a product to market. Brazil’s trade deficit in pharmaceuticals is R$6 billion per year. We need to make a public effort so that Brazilians develop drugs here,” Pagani says.
The implementation of alternative methods depends on the existence of laboratories recognized in the so-called good laboratory practices or GLP, but they are still few and far between in Brazil. “Good practices help with tracking and as a result they make the study more reliable. The reliability of alternative methods will also be ensured by making comparisons among the Renama laboratories,” says the coordinator of Concea, José Mauro Granjeiro, who is in charge of this area at Inmetro. Recently, Inmetro coordinated a comparison among five laboratories in the network with support from an experienced international consulting firm – the European Center for the Validation of Alternative Methods (ECVAM). The results are now being analyzed.
In order to expand studies of alternative methods, an effort to fund the groups of researchers that are involved will be required, observes Luiz Henrique Canto from the MCTI. “We have been able to design a structure and are beginning to make progress. The MCTI is making an all-out effort, including going before Congress for support for budget increases to strengthen Renama. We believe that this initiative may be of substantial benefit to the scientific and technological development of Brazil in the area of life sciences,” he says.
Republish