 EDUARDO CESARThe first surgery to implant an artificial heart developed in Brazil should be performed later this year. This is the expectation of those responsible for making the device, a team of doctors, engineers, technologists, biomedical experts from the Dante Pazzanese Institute of Cardiology in São Paulo, the Polytechnical School of the University of São Paulo (USP), the State University of Campinas (Unicamp), São Judas Tadeu University, Armando Alvares Penteado College, the Institute of Aeronautical Technology (ITA), São Jose dos Campos and the Sorocaba College of Technology. The device has passed all all of the bench tests and animal trials and is only awaiting approval by the Ministry of Health to begin implanting the artificial heart in humans. Named as coração artificial auxiliar, or “auxiliary artificial heart” (AAH), it is designed to be implanted in the chest of patients with severe heart failure that are in line for a real heart transplant. There is a fundamental difference in the two models of total artificial hearts (TAH) being used in the world today. The Abiocor and Syncardia, used in North America, are already implanted in about 100 patients, and involve the complete removal of the patient’s heart.
EDUARDO CESARThe first surgery to implant an artificial heart developed in Brazil should be performed later this year. This is the expectation of those responsible for making the device, a team of doctors, engineers, technologists, biomedical experts from the Dante Pazzanese Institute of Cardiology in São Paulo, the Polytechnical School of the University of São Paulo (USP), the State University of Campinas (Unicamp), São Judas Tadeu University, Armando Alvares Penteado College, the Institute of Aeronautical Technology (ITA), São Jose dos Campos and the Sorocaba College of Technology. The device has passed all all of the bench tests and animal trials and is only awaiting approval by the Ministry of Health to begin implanting the artificial heart in humans. Named as coração artificial auxiliar, or “auxiliary artificial heart” (AAH), it is designed to be implanted in the chest of patients with severe heart failure that are in line for a real heart transplant. There is a fundamental difference in the two models of total artificial hearts (TAH) being used in the world today. The Abiocor and Syncardia, used in North America, are already implanted in about 100 patients, and involve the complete removal of the patient’s heart.
“Our artificial heart is the first of its kind as it is conceived to work with the natural organ,” says Aron José Pazin de Andrade, a mechanical engineer who specializes in bioengineering, and professor and coordinator of the Center for Circulatory Assistance Engineering at the Dante Pazzanese Institute. In early June he attended the annual conference of the American Society for Artificial Internal Organs (ASAIO) in Washington to present the news. For the cardiologist and former Minister of Health, Adib Jatene, “with the auxiliary artificial heart created by Aron and his team, Brazil is taking over a new area of technology – and this is very important for the country. We will no longer be dependent on other nations in this sector.”
“The importance of the device, with research funded by FAPESP in the form of scholarships, the National Council for Scientific and Technological Development (CNPq), the Heart Hospital and Adib Jatene Foundation, can be seen when looking at the statistics of deaths related to cardiovascular disease. Heart disease is the leading cause of death in the country at about 300, 000 heart related mortalities per year, and the World Health Organization predicts that the index of these diseases is expected to increase as much as 250% by 2040. In many cases, the only form of treatment is to receive a heart transplant. In 2009, some 300 heart transplants were performed in Brazilian hospitals and thousands of people waited in line for the procedure. It happens that many of these patients die before they are submitted to the procedure, due to their advanced stage of heart disease. The artificial heart will help serve as a “bridge to transplant.” Aron Andrade believes that their artificial heart can support the patient for a minimum of 30 days and not longer than one year. Everything will depend on the condition of the natural heart and obtaining a donor organ.
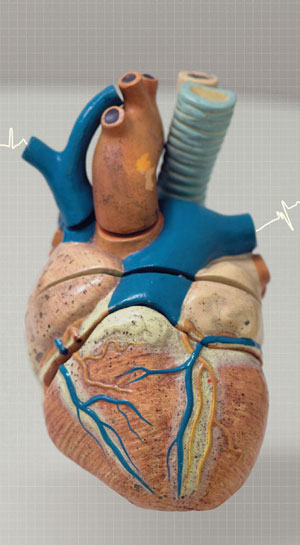
A little bigger than a tennis ball and weighing about 600 grams, the AAH is constructed with biocompatible materials such as polymers and titanium alloys. It is a pulsatile flow device that pumps blood only when the artificial ventricle fills. The operating principle of the AAH is electromechanical, equipped with two ventricles, the right one helps its counterpart in the real heart by sending blood with more pressure to the pulmonary artery, while the left, also coupled to its natural counterpart, pumps blood to the aorta, which then distributes it to the body. “In short, the natural ventricle pumps blood into the ventricle artificial ventricle, which pumps it out,” says Andrade. A battery powered motor drives the displacement action of diaphragms to produce a pulsating flow of blood, thus reproducing the functions of the natural organ. The device is implanted in the patient’s belly, below the diaphragm, and an electric cable, the thickness of a finger, exits the abdomen and conects to a control device that is responsible for commanding the operation of the AAH.
 The researchers expect the cost for each unit to be between $30,000 and $60,000. The final price will depend on demand and whether or not the Dante Pazzanese Institute partners with a private company for production. Following approval by the Ministry of Health, the artificial heart will be implanted into patients of the institute free of cost. Andrade believes that if all goes well with these first human trials, the procedure can soon be offered to patients of the Health System (SUS). The new device, explains the coordinator of research, offers some advantages over artificial units that completely replace the natural heart. The first advantage is that the surgery is simpler, faster and avoids the high-risk procedure in which the patient’s heart completely stops beating for a few hours and its normal function is replaced by a bypass machine. In addition, by sustaining the function of a patient’s natural heart, the blood pressure and pulse frequency of the artificial heart can be more accurately and easily controlled, which further contributes to the probability of success using this technique. The first implants of the CAA will be paracorporeal (outside the body), with the only direct connection being to the artificial ventricle, the left in this case, which is responsible for pumping blood from the heart to rest of the body. The left ventricle is generally the most damaged area of the heart in cardiac patients because it is the part making the greatest effort in circulating blood through the body. In a second phase, which should take place one year after the first procedures with the new heart are conducted, the team will begin to insert the implant in the abdominal cavity and engage the coupling of both artificial ventricles to the patient’s real heart.
The researchers expect the cost for each unit to be between $30,000 and $60,000. The final price will depend on demand and whether or not the Dante Pazzanese Institute partners with a private company for production. Following approval by the Ministry of Health, the artificial heart will be implanted into patients of the institute free of cost. Andrade believes that if all goes well with these first human trials, the procedure can soon be offered to patients of the Health System (SUS). The new device, explains the coordinator of research, offers some advantages over artificial units that completely replace the natural heart. The first advantage is that the surgery is simpler, faster and avoids the high-risk procedure in which the patient’s heart completely stops beating for a few hours and its normal function is replaced by a bypass machine. In addition, by sustaining the function of a patient’s natural heart, the blood pressure and pulse frequency of the artificial heart can be more accurately and easily controlled, which further contributes to the probability of success using this technique. The first implants of the CAA will be paracorporeal (outside the body), with the only direct connection being to the artificial ventricle, the left in this case, which is responsible for pumping blood from the heart to rest of the body. The left ventricle is generally the most damaged area of the heart in cardiac patients because it is the part making the greatest effort in circulating blood through the body. In a second phase, which should take place one year after the first procedures with the new heart are conducted, the team will begin to insert the implant in the abdominal cavity and engage the coupling of both artificial ventricles to the patient’s real heart.
Long road
Research to create of the artificial heart first began 15 years ago, when Andrade went to study this type of device in the United States at the Baylor College of Medicine in Houston, as part of his doctorate in the College of Mechanical Engineering at Unicam . The U.S. institution is a pioneer in the development of artificial hearts and heart transplants. “At that time, their device was not fully functional, but had already been implanted in animals,” he recalls. “When I returned to Brazil in 1997, I decided to continue this line of research, employing the same operating principle as the device that I studied in Houston. I had a cooperative agreement with the U.S. institution and three years later we realized the first bench tests.” In 2001, we began testing the new implants on animals, initially experimenting with the device outside the body of sheep. “These tests were exciting to us, because they showed that the device was truly feasible and, when connected to the animal’s natural heart, they functioned at the same pace. We then started to develop an artificial heart that could be directly implanted into the body and began performing experiments in healthy lambs, weighing between 80 and 100 pounds.” Only in 2010, after the successful testing of implants in six calves, was the efficacy of the device proven. It was at this point that approval for testing the device in the first human subject was solicited by the Ministry of Health.

EDUARDO CESARDante’s Heart: two ventricles EDUARDO CESAR
The search for circulatory assisting devices is a challenge faced by companies and institutions both in Brazil, and abroad. In Germany, for example, Dualis MedTech is working on a biventricular pulsatile flow pump similar to the artificial auxiliary heart developed at the Dante Pazzanese Institute. In Brazil, the Heart Institute at the College of Medicine at the University of São Paulo (InCor-HC/FMUSP) was responsible for creating the first artificial ventricle in Latin America, implanted in 1993 in a 30 year old patient in the terminal phase of Chagas disease. Designed and developed by bioengineering team at Incor, the artificial ventricle was connected to the patient’s left ventricle, so that he could wait for five days for a donor heart to become available. The difference between the artificial heart of Dante Pazzanese and the artificial ventricle of InCor is that the former consists of two artificial ventricles in one unit to better simulate the anatomy of a real heart.
“The InCor device can assist the right and left ventricles at the same time, or only one or the other of them, depending on the condition of the heart patient. These devices are connected to the heart and implanted in the abdominal region. Thirteen patients received the device while awaiting a real heart transplant for periods of 5 to 42 days, “says Professor Idágene Cestari, director of the Center for Biomedical Technology of InCor. According to the specialist, to assess the effectiveness of the artificial ventricle, InCor is currently coordinating a multicenter study supported by the Ministry of Health and CNPq, which are part of the National Institute of Cardiology of Rio de Janeiro, the Heart Hospital of the Federal University of São Paulo (UNIFESP), the Messejana Hospital in the state of Ceará, the Institute of Cardiology of Rio Grande do Sul and the Dante Pazzanese Instituto itself.
Rotation of the turbine
Another important category of circulatory assistance devices is the continuous flow blood pump, which is often used in cardiac patients because of their availability in the market, low cost and ease of implantation. Linked to both ventricles, these devices help the damaged heart to pump blood continuously and can be one of two different types: axial or centrifugal. In the former type, blood is pumped by a small turbine, similar to a boat propeller, that spins at a very high speed (10,000 revolutions per minute) and supports constant flow in the direction of the incoming blood. There are also centrifugal type pumps, which are larger than continuous flow devices, have blades that spin at a much slower rate of speed and generate a source of flow that is perpendicular to the direction of the incoming blood. Today, several centers around the world are studying this type of device, including Pazzanese.
Another relevant research program in the field of circulatory support devices being conducted in Brazil, is coordinated by Professor José Roberto Cardoso, director of the Polytechnical School (Poli) at USP. In a thematic project financed by FAPESP, drafted in the wake of experimental success with the artificial heart of the Dante Pazzanese Institute, researchers, with the participation of Professor Aron, want to develop a different model of a ventricular assistance device which employs a sophisticated electric motor that drives the pump’s propeller without actually coming into direct contact with blood. To this end, the research group is creating a magnetic bearing rotor that will function by means of levitation, driven by magnetic forces.
An important part of the device, the bearing works as a support that keeps the rotor in a fixed central position, allowing its rotation. According to Professor Oswaldo Horikawa of Poli, who is also part of the team, the goal of the magnetic bearing is to minimize the risk of physical injury to blood cells – a process called hemolysis – due to the force of pumping action. The device is currently in final assembly stages of a prototype model.
Project
Implantable electromagnetic propulsion systems for uni or biventricular artificial heart blood circulatory assist devices (nº 06/58773-1); Modality Thematic Project; Coordinator José Roberto Cardoso – USP; Investment R$ 1,185,540.09 and US$ 281,960.21 (FAPESP)