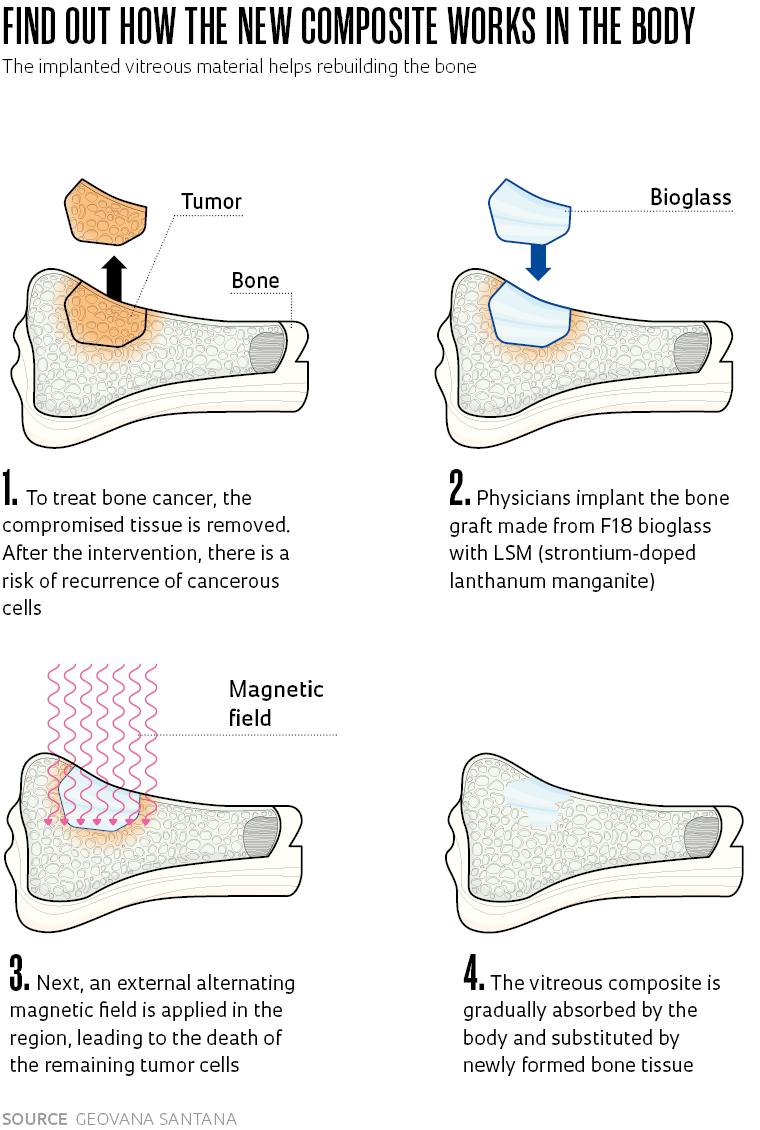
Materials engineer Geovana Lira Santana, from the state of Paraíba, discovered the properties of F18, a bioactive glass capable of stimulating bone regeneration, when she arrived at the Federal University of São Carlos (UFSCar) to do a master’s degree. She saw in the new material the possibility of realizing an old desire of becoming a scientist and doing research on cancer. From F18, a creation of the Vitreous Materials Laboratory (LAMAV) of the Department of Materials Engineering (DEMA) at UFSCar, Santana worked on the development of a compound with magnetic particles to treat bone cancer.
With collaboration from the Center for Research, Teaching, and Innovation in Glass (CERTEV) and the Functional Materials Development Center (CDMF), two Research, Innovation, and Dissemination Centers (RIDCs) funded by FAPESP, Santana developed a new material to be applied as a graft on bone affected by cancer. Into the glass matrix formed from the F18 bioactive glass (or bioglass), patented by UFSCAR in 2015, she incorporated doped lanthanum manganites, i.e., enriched with strontium, a material that heats up when exposed to an external alternating magnetic field.
The result was a composite—a material formed from two or more components with complementary or superior properties to those of the original items—with a dual function. The first is combating tumor cells through controlled heating of the magnetic particles; the second is the regeneration of bone tissue, as a result of the osteoinduction capacity of the bioglass. “The bioactive glass frees ions that change the pH of the medium, stimulating the proliferation of bone cells. It not only creates a favorable environment for bone regeneration, like the bone substitutes available on the market, but it also promotes the formation of tissue,” summarizes the researcher, who is currently doing a PhD in the same department. Additionally, F18 bioglass has a strong bactericide action which prevents postsurgical infections.
Started in 2018, the project had guidance from materials engineer Edgar Dutra Zanotto, coordinator of LAMAV and CERTEV, and collaboration from Murilo Crovace, professor from DEMA. Preliminary results, published in the scientific periodical Materials in 2022, are promising.
Another relevant feature of the new material is that it can be heated to a maximum of 45 degrees Celsius (°C). Therefore, it avoids overheating of the area and damaging healthy cells neighboring the tumor. In laboratory tests, the magnetic particles of the composite reach 40 oC in a few minutes when submitted to an external magnetic field.
“It is very close to the ideal temperature for treatment of the tumor, around 43 °C,” says Santana. “The formation of a layer of hydroxycarbonate apatite, naturally present in human bone, permits the connection of the composite with the bone tissue,” explains the researcher. Besides seeking the ideal heating levels, the next stages of the project cover carrying out in vitro testing and clinical studies, still with no scheduled date. An application to patent the composite was filed in 2021.
Medical radiologist Marcos Roberto de Menezes, coordinator of the area of radiology and image-guided intervention of the Institute of Cancer of São Paulo (ICESP), assesses that, once validated by clinical studies, the new material may bring new perspectives to oncology treatment. Menezes is a specialist in the treatment of cancer by thermoablation, a technique that consists of the image-guided insertion of needles to destroy tumor cells by increasing temperature or freezing.
According to the radiologist, the use of temperature as a therapeutic resource in oncology could be a less invasive alternative to surgery in specific cases, such as the treatment of metastasis. “Hyperthermia [a sharp increase in body temperature] therapy is already well established for tumor cell destruction. The big advantage of this new material would be the possibility of destroying the tumor and maintaining the structure and function of the bone,” evaluates Menezes. He explains that hyperthermia treatment destroys the tumor, but depending on the size and severity of the lesion there may be weakening of the affected bone, causing loss of function and pain. The osteoinduction capacity of the bioactive glass could solve this issue.
Even missing various stages before arriving on the market, the bioactive glass with magnetic particles from UFSCar already has a company interested in selling it: the startup Vetra, founded in 2014 by former CERTEV students and incubated in Supera, the business park for innovative companies of Ribeirão Preto.
The bioglass used as the matrix for the composite developed by Santana was a result of the master’s project of dentist Marina Trevelin, founding partner of Vetra, carried out in LAMAV-DEMA-UFSCar between 2009 and 2011. For her PhD, the researcher expanded the project advancing to preclinical trials and exploring different uses for the technology, such as the regeneration of wounds of the skin and nerves.
Trevelin received a FAPESP grant to carry out her PhD in materials science and engineering and Vetra had funding from FAPESP’s Innovative Research in Small Businesses (PIPE) program, for the industrial production of F18. The startup holds the patent for the material, licensed by UFSCar in 2016 (see Pesquisa FAPESP issue no. 241), and today provides it for companies specialized in medical and dentistry products.
Santana’s project is the successor of an even older story that dates back to 1977, when Zanotto created LAMAV at UFSCar. At that time, bioactive glass was still new in Brazil. It had been invented less than 10 years earlier, in 1969, by US materials engineer Larry Hench, of Florida University, in the USA, from a composition of sodium, calcium, silicon, and phosphorus.
The new material gained attention for its capacity to react with bodily fluids forming a layer of hydroxycarbonate apatite, which enables it to chemically bond to bone tissue and promote its regeneration. It was patented as Bioglass 45S5, a name which would popularize similar materials with different compositions.
In little time, bioglass gained prominence on the biomaterials market. “In Europe and in the USA, it has been used to produce bone grafts, membranes for the regeneration of skin ulcers, and, in powdered form, dental products for repairing defects in the enamel and the treatment of dentin hypersensitivity,” highlights Zanotto.

Léo Ramos Chaves / Revista Pesquisa FAPESPSamples of bioglass with different compositions of magnetic materialLéo Ramos Chaves / Revista Pesquisa FAPESP
However, the material invented by Hench presented limitations due to its low mechanical resistance. This characteristic prevents its use as an implant in locations subjected to large loads and limits the capacity of molding it into different formats. One day, during an informal chat at the table of a bar, Zanotto and Hench speculated about these limitations when the idea of a new project arose. “We imagined that it would be possible to crystallize the bioglass to give it greater resistance,” recalls the Brazilian. This project ended up being the topic of Oscar Peitl’s materials engineering PhD, today a professor at DEMA-UFSCar. From this research, concluded in 1995, biosilicate was born and later patented in 2003.
Zonotto explains that biosilicate is a vitroceramic (see Pesquisa FAPESP issue no. 191). In the crystallization process, done through the insertion of additives and exposure to high temperatures, the material, which initially has a disorderly structure, comes to have a regular geometric arrangement. The crystallinity confers the vitroceramic superior mechanical properties than bioactive glasses, but generally reduces the bioactivity index. Therefore, the challenge for the UFSCar team was to design a formula that would provide bioactivity similar to that of bioglass while maintaining the high mechanical resistance. The result pleased the researchers.
“In the past years we have conducted three different clinical studies with biosilicate, all successful,” informs the coordinator of LAMAV. According to Zanotto, besides being efficient in the treatment of dentin hypersensitivity, in powdered form, the material has enabled the production of implants of the middle ear ossicles and an ophthalmic implant. This glass ocular prosthesis presents movement and appearance very similar to that of the natural eye.
Years later, in the creation of F18 bioglass, the researchers had wanted the opposite result to biosilicate: that it did not crystallize when subjected to high temperatures, enabling greater control of the material in the production of fibers, vitreous tissues, and complex 3D parts. Trevelin says that 17 attempts were made until the ideal formula—F18—was obtained. “We arrived at a composition that was stable and highly bioactive, as well as being bactericidal. Since the start of the preclinical trials, in 2011, F18 has shown promise in tissue regeneration,” says Trevelin.
Taking advantage of the bactericidal properties of F18, Trevelin, of Vetra, intends to employ it as a bone graft in patients affected by osteomyelitis, a bone disease caused by pathogenic microorganisms. She has already begun research in this direction, with funding from PIPE. Conducting clinical trials now depends on investments into the infrastructure of the startup, which the researcher expects to do with the aid of PIPE Invest, a modality of support for startups and small and medium businesses.
At the Federal University of ABC (UFABC), in Santo André, São Paulo, a research group led by materials engineer Juliana Marchi has also developed a vitreous composite to fight bone cancer using hyperthermia. Besides magnetic nanoparticles to heat the affected region, the composite material has two other therapeutic agents: the chemical element holmium (Ho) for application of brachytherapy, a type of internal radiotherapy for destruction of the tumor, and zoledronic acid, a drug used for treating bone metastasis.
According to Marchi, this multidisciplinary approach allows the development of a new methodology, described in an article published in the journal Biomaterials Advances, in 2022, which resulted in a glass with high bioactivity and magnetization. One differential of the material is the possibility of dosing the rate of delivery of the therapeutic agents associated with the composite. “We can modulate the dose of brachytherapy at the time the bioactive glass containing the incorporated radioactive property of holmium is implanted,” she explains. The project, funded by FAPESP, is in the in vitro testing phase.
Projects
1. Development and characterization of highly bioactive flexible vitreous tissues (no. 11/22937-9); Grant Mechanism Doctoral (PhD) Fellowship; Supervisor Edgar Dutra Zanotto (UFSCar); Beneficiary Marina Trevelin Souza; Investment R$168,950.29.
2. Development of a methodology for producing high-purity particulate bioactive glasses on an industrial scale (no. 15/17175-3); Grant Mechanism Innovative Research in Small Businesses (PIPE) Principal Investigator Marina Trevelin Souza (Vetra); Investment R$600,150.88.
3. CEPIV — Glass Teaching, Research, and Innovation Center (no. 13/07793-6); Grant Mechanism Research, Innovation, and Dissemination Centers (RIDCs); Principal Investigator Edgar Dutra Zanotto (UFSCar); Investment R$47,014,754.96 (for all projects).
4. Development of an injectable multifunctional composite for treating bone cancer with hyperthermia and brachytherapy associated with bone repair (no. 20/00329-6); Grant Mechanism Regular Research Grant; Principal Investigator Juliana Marchi (UFABC); Investment R$166,899.74.
5. Development of bioglass-based bactericidal putty for use as a bone graft in patients with osteomyelitis (no. 20/09584-9); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Marina Trevelin Souza (Vetra); Investment R$107,174.35.
6. Absorbable grafts with bioactive properties for complex bone reconstructions (no. 22/11291-5); Grant Mechanism Innovative Research in Small Businesses (PIPE); Agreement FINEP PIPE/PAPPE; Principal Investigator Diogo Ponte Lauda (Selaz); Investment R$763,130.20.
7. Absorbable grafts with bioactive properties for complex bone reconstructions (no. 18/22486-6); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Karen Julie Santos Gracianinov Costa (Selaz); Investment R$1,437,209.97.
Scientific articles
SANTANA, G. L et al. Smart bone graft composite for cancer therapy using magnetic hyperthermia. Materials. apr. 2022.
BORGES, R. et al. Superparamagnetic and highly bioactive Spions/bioactive glass nanocomposite and its potential application in magnetic hyperthermia. Biomaterials Advances. apr. 2022.
Republish