
Léo Ramos Chaves
Kleppner: “From the point of view of psychological balance, it is good to teach and to research”Léo Ramos ChavesInitially at Harvard University, from 1959 to 1966, and since that time at the Massachusetts Institute of Technology (MIT), the American scientist Daniel Kleppner has led or participated in experiments that established three techniques which today are widely used in physics. The first is the atomic clock that uses a hydrogen maser (microwave amplification by stimulated emission of radiation), which resulted in the Global Positioning System (GPS) that today is used in cars and cell phones. The second is cavity quantum electrodynamics, which enabled the study of quantum properties such as the phenomenon of entanglement, in which any action on a particle can affect its partner, even if it is distant. The third technique is the containment and cooling of atoms to produce Bose-Einstein condensates, a state of matter obtained at near absolute zero (-273°C) which was predicted by the Indian physicist Satyendra Bose (1894–1974) and by Albert Einstein (1879–1955) and demonstrated experimentally in 1995.
Kleppner, who in January received the American Physical Society’s Medal for Exceptional Achievement in Research, grew up in New York. The son of an Austrian immigrant, he enjoyed building crystal radios and small planes as a child. After graduating in engineering in 1953, he studied for two years at Cambridge University in England, before moving on to Harvard. There, Kleppner and his doctoral supervisor, Norman Ramsey (who won the Nobel Prize in Physics in 1989), developed the hydrogen maser; this atomic clock was 100,000 times more accurate than those which had been used to measure time up to that point.
In 1989, Kleppner was at a restaurant in the city of São Carlos, São Paulo, with Brazilian colleagues when he wrote down the ideas for his debut article as a columnist for Physics Today, the journal of the American Physical Society (APS). In the article, titled “A passion for precision,” he described the pleasure he found in seeking new methods to measure the properties of the atoms he discovered with Ramsey. He wrote for Physics Today until 2013.
Since 1985, he has made a number of visits to the São Carlos Institute of Physics at the University of São Paulo (IFSC-USP), with which he continues to collaborate. He is 84 years old, and has three children and four grandchildren with his wife Beatrice, a high school teacher. He returned to Brazil in February of this year to visit his former colleagues and to deliver the master lecture “Three Seeds in the Flowering of Quantum Sciences.” In this address, he looked back at his participation in the study of atomic clocks, his in-lab production of Rydberg atoms (atoms with electrons that have so much energy that they can be more than 10,000 times farther from the ion core than normal electrons), and Bose-Einstein condensates (see Pesquisa FAPESP, issue nº 101). “The students were very enthusiastic, as were the research groups I visited at the Institute of Physics,” he commented. There, two physicists he advised are now professors: Jarbas Castro Neto and Vanderlei Bagnato. In this interview two weeks after his presentation, Kleppner returned to the circumstances and difficulties of each of these projects.

Ryan K Morris / Bloomberg News
Kleppner, alongside then-president of the United States, George W. Bush, receiving the National Medal of Science in 2007…Ryan K Morris / Bloomberg NewsHow did you begin your career in atomic physics?
I had a great physics teacher in high school, and wonderful professors in college. At the University of Cambridge, where I spent two years as a grad student, my tutor Kenneth Smith mentioned an article proposing a type of clock that would be accurate enough to test Einstein’s predictions about the effect of gravity on time. The idea that gravity could affect the way a clock worked and the very passage of time seemed disturbing. I didn’t do anything immediately, but the idea stayed in my mind. Later I went to Harvard University and joined the group led by Norman Ramsey (1915-2011). He was the one who invented the technique that made atomic clocks useful in practice.
What did Ramsey do?
He imagined a type of clock that did not function using a cesium beam, which had been the initial standard, but rather used what later became known as the hydrogen maser. An atomic clock, as it had been used, has a beam of atoms that responds to radiation with a certain frequency. The response of the atoms is used to control the frequency of an electronic oscillator. In a maser, the atoms in a beam of molecules are filtered when they enter a cavity, where after a while they all begin to emit radiation, the oscillations of which can be measured. I joined Ramsey’s group just as they were considering how to do this, which at the time seemed impossible. Norman believed that placing the atoms in a resonant cavity could possibly increase the accuracy of atomic clocks by a factor of one thousand. For my doctorate, I built and tested a device that proved promising; later, together with a graduate student, I built the maser, which began to operate in the following year. In the late 1950s, Ramsey and I started to develop a maser that would go into space. The Space Age had begun, and NASA was very interested in testing Einstein’s theory with a satellite. But we started to get concerned.
Why?
Our objectives at Harvard and theirs at NASA were not exactly the same. NASA insisted that the astronauts have an active role in the experiment, but when a clock begins to operate, the best thing to do is to leave it alone. I was also concerned about another problem. What if we found a result that differed from Einstein’s theory? We could repeat the experiment, but it would take many years and there would be major questioning of Einstein’s work. We decided not to, but one of the researchers in the group who worked with a company interested in making the clock a commercial product liked the idea, and continued to work with the United States Navy. They did a much better experiment than what we had planned. Instead of putting the clock on a satellite, they placed it in a rocket that reached a height approximately the same as the earth’s diameter in space, and returned. The experiment confirmed Einstein’s theory, led to advances in the technology for hydrogen masers, and helped adjust the techniques for comparing clocks in space with those on Earth. An offshoot of this work was the Global Positioning System, which works by comparing clocks in space with those on the ground. Just think: the idea of confirming Einstein’s theory of general relativity led to GPS. We didn’t develop GPS, but atomic clocks are at the heart of it. For me, this is a fine example of how basic research provides unexpected rewards.

Personal Archive
…and with his mentor Norman Ramsey (center) and his former student William Philips (at right), both winners of the Nobel Prize in PhysicsPersonal ArchiveHow have atomic clocks evolved?
Since they were created in the 1990s, their accuracy has multiplied by 100,000. Until 10 years ago, all clocks functioned only in the microwave frequency, at 109 cycles per second. A new technology using optical frequencies, with cycles that are 10,000 or 100,000 times faster, worked very well. But now, what will we do with these clocks that are so much more accurate? The gravitational effect on time is no longer something interesting to observe. Someone could change the game, and use the clocks to measure gravity. This is only speculation, but measuring the variation in gravity on Earth with this precision could give an immediate view of transformations in the earth’s geology and in the oceans. This could be important, because of climate change.
Your laboratory was one of the first to create Rydberg atoms [with electrons more than 10,000 times farther from the nucleus than normal electrons]. What was that like?
Other groups did it practically at the same time. This idea came back and forth in my mind for years. At Harvard, a very creative, deep, and pleasant physicist, Edward Purcell (1912-1997), who was one of the inventors of nuclear magnetic resonance, told me about a discovery in radio astronomy. Some researchers had seen evidence of hydrogen atoms that had just been formed in a nearby star. In this star, protons and electrons united to form a hydrogen atom, which is only one proton joined to an electron. But the electrons had connected at a very large distance, and descended from one orbit to another until they reached a state of lower energy. I thought about how beautiful this was, and started to examine the extraordinary properties of these atoms. Their states are characterized by what is called the principal quantum number, n. Normally, n is a small number, 5, 4 or 3. In that case, it was n=100. Then the astronomers were able to see a signal emitted when the electron went from n=100 to n=99. Very low density would be required to see these states, and this requires a very large volume, like in space. In the early 1970s, we saw that we could produce these atoms in the laboratory using lasers [since they are large and easy to detect, Rydberg atoms could be manipulated and analyzed more easily than normal atoms]. It worked on the first try! That was my only experiment that worked on the first try.
You were also one of the first to produce Bose-Einstein condensates in the laboratory.
An article in Physical Review Letters in 1976 summarized what we knew about the physics of hydrogen atoms, and ended with a very interesting observation. The authors, William Stwalley of the University of Connecticut and Lewis Nosanow of the Materials Research Division, one of the centers maintained by the National Science Foundation (NSF), said that if atomic hydrogen could be arranged in a particular state, it could be cooled until absolute zero but never would turn into a solid or liquid. Hydrogen is much lighter than helium, and has so much energy that it doesn’t become a liquid, even at absolute zero. But if it is cooled enough, this hydrogen gas could undergo a change, a Bose-Einstein condensation. I read the article, but I put it aside because to subject hydrogen to these temperatures and density seemed absurd. I told this to my MIT colleague Thomas Greytak, who knew a lot about liquid helium, and he explained what Bose-Einstein condensation was, something I had never heard of. We finally realized that this was a new world at low temperatures, and that perhaps the experiments would work. In the early 1980s, we were initially successful, but we found that at high densities the atomic hydrogen was transformed into molecular hydrogen [forming pairs] and disappeared. Finally in 1998, Greytak and I managed to make the condensate with hydrogen, although today we know that this is not the best atom to do this with. Creating these quantum gases with the cooling technique that today uses lasers opened up a whole new world to physics.
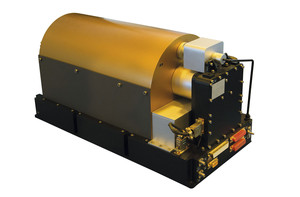
SkywalkerPL
Hydrogen maser on board the Galileo satellites, the global navigation system of the European Union; measures time accurate to billionths of a secondSkywalkerPLIn addition to research, you have always been very interested in teaching.
Teaching and research go hand in hand. There is a psychological advantage. Because sometimes, experiments go wrong and we get very upset. It is always a consolation to think: “Well, I’m still a teacher!” And, of course, sometimes a class goes wrong, we feel depressed, but we can say: “But I do research!” From the point of view of psychological balance, it is good to have these two aspects. And when we explain something to students, we are also explaining it to ourselves. Teaching should be a creative process, to find new ways of understanding things, which is also part of the work of scientific research.
Do you still work in the laboratory?
No, but I still have my office at MIT. I go there several days a week. I like being there. MIT has a wonderful atmosphere, my friends are there, there are excellent lectures. But I no longer do research. In one of my essays for the Reference Frame section, published in Physics Today in 1998 under the title “Nibbling the bullet,” I wrote that people should retire. In the United States you do not have to retire, but I believe that researchers should do so, in order to make room for younger researchers, among other reasons. I wrote the essay when I was 65, suggesting that 70 would be an appropriate age to retire. Then I realized that I had made a public commitment! And that is what I did. What I did not notice at the time was how fast those last five years would go… Although I’m retired, I am still active at MIT. If I wanted to participate in research and there was space, I would be able to do so, but the laboratories are occupied by younger staff. It’s been a good life.