
Léo Ramos Chaves/Pesquisa FAPESP MagazineA researcher inserting a solution into a biosensor, with a prototype troponin measurement unit in the backgroundLéo Ramos Chaves/Pesquisa FAPESP Magazine
The leading cause of death worldwide, cardiovascular diseases, and in particular heart attack and stroke, cause 17 million deaths annually according to the World Health Organization (WHO). In Brazil, around 400,000 people die each year as a result of heart disease. Being able to rapidly diagnose patients who present with heart attack and stroke symptoms is crucial to saving lives or avoiding sequelae.
This led researchers at Cor.Sync, a healthtech firm initially founded in Curitiba and now based in São Paulo, to develop a system capable of measuring blood levels of cardiac troponin—the primary biomarker for detecting heart attacks—in less than 10 minutes. With current best available technology, doctors have to wait as much as four hours to get the test results back. The new solution, which is in the final stage of clinical trials, was developed with FAPESP support via the Innovative Research in Small Businesses (PIPE) program.
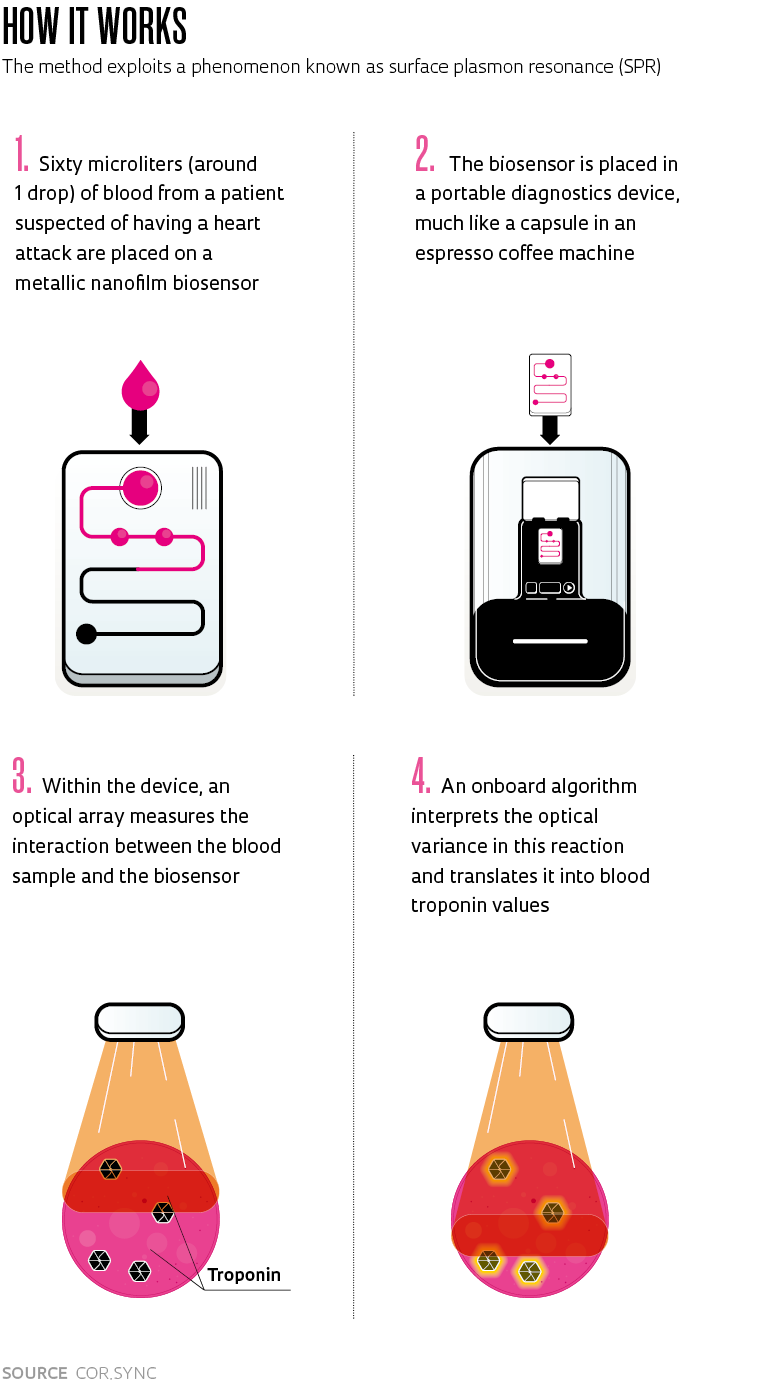
“Our method uses high-specificity biosensors to measure the concentration of cardiac troponin in the patient’s blood,” explains Raul de Macedo, Cor.Sync’s founder and CEO. In addition to the new test method, the company has also designed a portable point-of-care device for on-the-spot blood testing.
“Troponin readings are shown within eight minutes on the display and in the patient’s electronic records, helping physicians quickly determine if they have suffered a heart attack,” says Macedo. Brazilian and global patent applications have been filed for both the method—which has been proven in bench-top studies—and the hardware.
The advantage of a point-of-care troponin test system is that blood testing can be done right in the emergency room without having to send a sample to the hospital lab. “We save precious minutes that can make all the difference in a patient’s treatment,” says cardiologist Attílio Galhardo, a partner at Cor.Sync and a member of the team that developed the new system.
Heart attacks are typically diagnosed by reviewing the patient’s clinical history and by performing an electrocardiogram (ECG), a test that measures the patient’s heart rate and shows any myocardial alterations resulting from ischemia—a reduction in blood flow through the heart—or necrosis. An ECG, however, is not sensitive enough to detect minor or short-lived myocardial injury. When available, a troponin blood test is considered the gold standard for diagnosing heart attacks.
“When an ECG is not conclusive, the physician will then request a blood test. But most hospitals in Brazil and around the world take two to four hours to get the results back from a blood test. Even at some of the country’s leading hospitals, test results are rarely available within less than an hour. That’s too long,” says Galhardo. According to the Brazilian Society of Cardiology (SBC), around 50% of heart attack victims will not present with significant changes on an electrocardiogram. “For these cases, troponin testing is crucial to get an accurate diagnosis,” argues Galhardo.
Lab tests to detect cardiac troponin are fairly accurate, with a tolerance of 7%—i.e. if the actual troponin value is 10 nanograms per liter (ng/l), the system will show a value ranging from 9.3 to 10.7 ng/l. This margin of error is deemed acceptable by the medical community. On the downside, these tests take several hours to perform, and require the sample to be handled by specialist personnel. “The test typically takes 40 minutes to run in the machine, not to mention the time needed to prepare the sample and transport it to the lab,” says Macedo.
There are several commercial point-of-care troponin testing systems now on the market that are similar to the one developed at Cor.Sync., says Macedo. “They are also able to measure troponin levels within minutes, but are not accurate enough for diagnostic purposes. For this reason, they are often used for triage pending a laboratory test for confirmation,” he says. “We have been able to combine the diagnostic accuracy of gold-standard lab tests with the speed and convenience of point-of-care diagnostics.”
Cardiologist Pedro Ivo de Marqui Moraes, who serves as an assistant physician in a cardiology Intensive Care Unit (ICU) at the Federal University of São Paulo (UNIFESP), says point-of-care troponin testing methods have been a game changer. “In an emergency-room or ICU setting, it is far more practical to have a device that can process the test and display the results at the bedside in a matter of minutes,” he says. “Laboratory troponin tests have been improved over the years but are becoming obsolete. We’re now transitioning to on-the-spot testing.”
Plasmon resonance
The method developed at Cor.Sync exploits a physical phenomenon known as surface plasmon resonance (SPR). A laser beam interacts with a metallic nanofilm biosensor onto which the blood sample is placed (see the infographic). “Commercial point-of-care troponin tests employ other methods. As far as we know, SPR has never been used for diagnostic purposes but only to monitor protein interactions in other applications. What we’ve done is adapt this method to a diagnostic platform,” explains Macedo.
The idea of creating a device to speed up diagnosis of heart attacks was first conceived in 2017, the year Macedo started his master’s degree in bioengineering at the Federal Technological University of Paraná (UTFPR). “I had recently felt pain in my chest and thought I was having a heart attack. I went to an ER and, fortunately, it turned out to be stress. But I noticed it took a long time to get the test results,” he recalls.
In 2019, he decided to create a startup in his hometown, Curitiba, to develop a commercial device for rapidly detecting troponin. The next year, he moved Cor.Sync to the Center for Innovation, Entrepreneurship, and Technology (CIETEC) at the University of São Paulo (USP) for incubation. The related research and development activity was mostly based in São Paulo. “I realized that developing a scalable commercial product would be too complex a task without the help of capable researchers. So we approached USP and signed a cooperation agreement with SisNano USP, a network of nanotechnology laboratories,” he says.
“Cor.Sync contacted us and we’re helping them develop the biosensor,” says chemist Koiti Araki, head of SisNano USP. “We took an interest in the project because of the potential there is in medical diagnostics, and because the method is based on a nanotechnology technique, surface plasmon resonance. The advantage of this approach is that it can detect even a very small number of troponin molecules in the blood sample.”
Cor.Sync’s diagnostic method has been validated in a bench-top study, with the method producing results at an accuracy within 7%, similar to gold-standard laboratory tests. The startup has also completed development of its first prototype point-of-care device—a second version is due to be completed in October.
The final stage of development—a trial with patients to clinically validate the methodology—is scheduled to be completed within the year. The startup is in final talks with a hospital in São Paulo to conduct a trial with 600 volunteers—patients arriving at the emergency department with a suspected heart attack. “We hope to publish our results toward the end of the year or early next year to support our application for a usage permit at ANVISA [Brazilian Health Regulatory Agency]. If everything goes as planned, our product should be on the market within 2023,” says Galhardo.
Cor.Sync received R$2.85 million in seed funding from private investors and nonrepayable funds. Alongside a grant from PIPE-FAPESP, the firm received funding from the Brazilian Funding Authority for Studies and Projects (FINEP), an agency linked to the Ministry of Science, Technology, and Innovation (MCTI); from Conecta Startup Brasil, a federal government initiative to advance entrepreneurship; and from the IA2 program, run by MCTI with support from Softex, an organization dedicated to enabling the digital transformation of Brazilian firms.
Project
Development and validation of a point-of care device for measuring cardiac troponin levels in blood (nº 20/05786-6); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Raul Queixada (Cor.Sync); Investment R$140,501.60.