 FABIO OTUBOThe two animated films Ghislain Saunier showed his volunteers in an experiment performed in 2008 at the Federal University of Rio de Janeiro (UFRJ) were no work of art. Lasting a few seconds, each sequence of images showed 10 small, white circles moving on a black background. In one, the movement of the circles formed figures that resembled a person walking—the white circles actually marked the joints of the legs, arms and trunk of someone filmed while walking. In the other, the circles moved randomly.
FABIO OTUBOThe two animated films Ghislain Saunier showed his volunteers in an experiment performed in 2008 at the Federal University of Rio de Janeiro (UFRJ) were no work of art. Lasting a few seconds, each sequence of images showed 10 small, white circles moving on a black background. In one, the movement of the circles formed figures that resembled a person walking—the white circles actually marked the joints of the legs, arms and trunk of someone filmed while walking. In the other, the circles moved randomly.
The two films, created by Saunier during his doctorate in the laboratory of neuroscientist Cláudia Vargas at UFRJ, were exhibited dozens of times, in a random order, to the 16 participants in the study. The volunteers watched the animations while an electroencephalogram machine recorded their brain’s electrical activity. In general, the participants recognized the first sequence of images quickly, but had difficulty in making sense of what they saw in the second film. With this experiment, Saunier and Vargas tried to find out if the brain’s processing of the two images differs significantly. “A key debate in neuroscience is understanding how the brain encodes and segments the objects in a visual scene through a combination of their attributes,” says Sergio Neuenschwander, a researcher at the Federal University of Rio Grande do Norte (UFRN) studying the response of small groups of neurons in animals exposed to different visual stimuli.
Today, experiments like this are increasingly common, beginning with recording the brain activity of animals and volunteers in the laboratory and continuing in a simple meeting room where neuroscientists, mathematicians and specialists in other fields discuss, with the aid of paper and pen, the best way to process, analyze and explain the data. Over the last few years, a three-story building at the Institute of Mathematics and Statistics, University of São Paulo (IME-USP) has become the headquarters of a multidisciplinary network that works along these lines: the Center for Neuromathematics or NeuroMat, one of 17 Research, Innovation and Dissemination Centers (RIDC) funded by FAPESP (see Pesquisa FAPESP Issue nº 208).
Coordinated by the mathematician Antonio Galves, NeuroMat brings together mathematicians, neuroscientists, physicians, physicists, computer scientists and statisticians to pursue an ambitious goal: to develop a new general theory of the brain, capable of explaining how the coordinated activity of a system composed of tens of billions of neurons and other cells can give rise to complex behaviors that enable interactions with a constantly changing environment. In addition to generating new abstract formulations for phenomena such as neuroplasticity (the ability of brain cells to reconnect), this line of research could have clinical impacts and enhance the methods for assessment and treatment of individuals with nervous system damage.
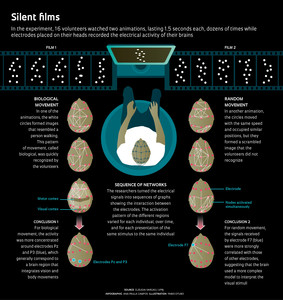 Infographic Ana Paula Campos Illustration: Fabio OtuboIn a workshop held in January, 2014 at NeuroMat, Vargas, one of the principal investigators at the center, and his collaborators in the project presented the latest analysis from the experiment started in 2008. This analysis used tools from an area of mathematics known as graph theory, and provided an opportunity to begin to observe how different areas of the brain interact under the two test situations: watching the video representing biological motion (a person walking) and watching the animation representing nonbiological motion (random circles).
Infographic Ana Paula Campos Illustration: Fabio OtuboIn a workshop held in January, 2014 at NeuroMat, Vargas, one of the principal investigators at the center, and his collaborators in the project presented the latest analysis from the experiment started in 2008. This analysis used tools from an area of mathematics known as graph theory, and provided an opportunity to begin to observe how different areas of the brain interact under the two test situations: watching the video representing biological motion (a person walking) and watching the animation representing nonbiological motion (random circles).
After the film sessions, years of work using different data interpretation strategies were needed to obtain the current results, also presented in January in the journal PLOS One. The electrical signals collected by Saunier did not allow immediate identification of significant differences between the brain’s functioning during the exhibition of the two films. Vargas and Saunier—in collaboration with researchers Thierry Pozzo, Elisa Carvalho Dias, José Magalhães de Oliveira and Eduardo Martins—made progress by adding up the outputs from the 20 electrodes each time participants watched one of the videos. This strategy showed that there was a slightly different brain activation pattern associated with each type of movement—biological and random. However, there were limitations.
“This manner of analyzing the data generated a fragmented description of neuronal activity, electrode by electrode, without offering a systemic view,” says Vargas. But it did not allow us to infer how different brain regions interact with each other in each situation. The challenge, then, was to develop a way to measure the interaction between brain areas.
Through NeuroMat, Vargas and his colleagues established a partnership with physicist Daniel Fraiman, of the University of San Andrés in Buenos Aires, who is a specialist in processing the brain’s electrophysiological signals. To interpret the data, Fraiman adapted a method previously employed with magnetic resonance data for use with electroencephalograph data. This new approach uses tools from graph theory, which studies the ways in which points or nodes in a network can connect. The main idea is to consider the electrodes as nodes in a network and connect the pairs of electrodes that tend to record signals during the same window of time.
Thus, they were able to construct graphs representing how the brain areas associated with the perception and control of body movements communicated among themselves and interpreted the animations of the original experiment. “This is an innovative way to use electroencephalography to map networks of interactions that reflect the activity of the brain in response to visual stimuli,” says Vargas, coordinator of the group that published the findings in PLOS One.
“Contemporary neuroscience is increasingly quantitative,” says Sidarta Ribeiro, director of the UFRN Brain Institute and a member of NeuroMat, which includes groups from the University of Campinas (Unicamp), Federal University of the ABC (UFABC), Federal University of Rio de Janeiro (UFRJ), the National Institute of Pure and Applied Mathematics (IMPA) and international centers. “We need to interact with mathematicians and statisticians of the highest level,” Ribeiro said.
Graph theory
The quantitative study of the organization of the brain—the connections between neurons and between different brain regions—has advanced in the last decade thanks to graph theory and the other areas of mathematics whose development is part of NeuroMat’s scientific project. Graph theory permitted analysis of the structure of what are known as functional brain networks—sets of distinct parts activated simultaneously during an activity, such as the perception of visual stimuli. The application of this theory to functional magnetic resonance images (fMRI) has produced evidence that functional brain networks appear to have a structure of the “small world” type, in which any two sets of neurons are connected through only a small number of intermediaries, and have only a few dozen hubs, meaning areas more connected with the rest of the network than others. “But the maps we have of brain connections still have a very low resolution, comparable to the world maps used in the sixteenth century,” says neurologist Marcio Balthazar, of Unicamp, who studies functional networks in patients with Alzheimer’s at the Institute of Research on Neurosciences and Neurotechnology, another RIDC focusing on neuroscience, coordinated by the neurologist Fernando Cendes, of Unicamp (See Pesquisa FAPESP Issue nº 215). Galves disagrees with that analogy. “The comparison with a world map is not accurate, because in the case of sailing, the shore beyond the sea actually existed; in the case of connection models of the brain, we work with theoretical constructs designed to represent, at a higher level of abstraction, a set of phenomena observed experimentally” he explains.
The functional networks related to the visual stimuli studied by Vargas’ team, for example, could be observed only when researchers were able to represent the electroencephalograph data as a graph. Thus, the researchers developed criteria to evaluate how the signal from each of the 20 electrodes was related to all the others as a function of time.
They were then able to construct a sequence of graphs, in which each one represented the network of interactions between the electrodes at 300 ms intervals. Simply visualizing these networks, however, revealed that they were highly variable. Not only did they vary between individuals and over time, they also varied between presentations of the same stimuli to the same individual (see infographic).
In order to find significant differences between the networks activated while viewing each video, a quantitative analysis of the connections of each node was required, measuring, for example, whether some of these were more connected with their neighbors or with the rest of the network than others in the random videos compared to the videos in which the volunteer was able to identify the biological image (a man walking).
One of the team’s findings was that, when the volunteers saw the randomly moving circles, brain signals detected by the electrode F7, installed just above the midpoint between the left eye and ear, correlated more strongly with other electrodes than in the case where the volunteers were watching the video representing a person walking. In the functional network resulting from the random movement video, it was also of interest that the activity of a region of the visual cortex near the O2 electrode, placed on the right side of the back of the head, communicated intensely with neighboring regions. It is as if the brain uses a more complex model when it cannot easily make sense of what is happening. The functional network activated when viewing the video representing a person walking was more concentrated around the electrodes Pz and P3—which generally correspond to a brain region that integrates vision and body movements—than that activated when viewing the random movement.
Gaps and solutions
The cooperation between neuroscientists and mathematicians in the experiment initiated by Saunier in 2008 at UFRJ continues, now with the challenge of finding mathematical models that would explain the variations and constancies in the sequences of graphs observed by the team. “Although highly variable, the sequences of graphs obtained during the processing of biological motion must have stable characteristics that are distinct from those observed in the processing of non-biological motion,” says Galves. “The scientific question is how to identify the set of stable characteristics within the variable sequence of the graphs obtained.”
The next step, he says, would be to apply a statistical procedure to select the mathematical models that best explain the sequences of graphs. He and his colleagues say, however, that these mathematical models do not yet exist and must be developed. The NeuroMat team imagines that these new models are what mathematicians call stochastic processes, evolution subjected to the influence of chance. These processes represent the overall activity of a system with many components interacting among themselves over time.
Galves and international collaborators, including the mathematician Eva Löcherbach, of the University of Cergy-Pontoise, France, have been working on developing a new class of stochastic processes that will allow identification of these regularities in complex interactive systems. In a 2013 article in the Journal of Statistical Physics, Galves and Löcherbach introduced, in simplified form, a new class of stochastic processes, generalizing properties of other, known classes, such as the fact that a next step in a chain of events is influenced by a variable number of previous steps, and presents qualitative behaviors compatible with empirical neuroscience findings.
Galves explains that the class of models under development at NeuroMat is not intended to provide detailed descriptions of brain function, but rather to identify regularities in neuronal interactions that are imperceptible in experimental data. According to the mathematician, the goal is to establish a new field within mathematics. “There is a naive view that what neuroscience needs is more data and more calculation power, but that alone is not enough,” says the center’s coordinator. “In fact, a conceptual framework is needed to formally express neurobiological phenomena.”
This and other efforts were discussed by NeuroMat members in São Paulo, in January, 2014, during the workshop in which they presented recent results of their research and planned upcoming activities. One of the participants, the neurologist Leonardo Cohen, from the National Institute of Neurological Disorders and Stroke, in the United States, considers NeuroMat’s proposal “very original.” Cohen is one of NeuroMat’s scientific advisors and spoke of his recent work on relearning movements lost after a stroke. “NeuroMat is quite different than initiatives underway in the United States and Europe.” Cohen believes NeuroMat has an advantage in that its main objective is not the collection of biological data, as is the case with the Project for Mapping Brain Activity, which intends to map the activity of each of the 86 billion neurons in the human brain. “In a project of that type, the work of the neuroscientists, engineers and computer scientists has been predetermined, and everyone knows what they need to do,” he explains. “NeuroMat, though, is like a school where people are beginning to explore connections among their specialties that they did not know existed.”
Neuroplasticity
At the workshop, Vargas and Cohen, one of the pioneers in the study of neuroplasticity in patients recovering from brain injuries, discussed a possible partnership between their teams. We want to understand the rules of neuroplasticity, what the rearrangement of functional networks makes possible or impossible,” says Vargas. “For this we need mathematical models that take into account that the brain is not deterministic; for example, our models and experiments need to consider that, with each repetition of a movement, the brain activates a network similar to the previous time, yet new in some aspects, which requires new conceptual and methodological instruments.” Galves believes that the key to mathematically describing neuroplasticity is learning to represent this evolution using stochastic processes that show groups of interactions between brain regions following injury or during motor learning.
Vargas currently uses electroencephalography and transcranial magnetic stimulation at the Deolindo Couto Institute of Neurology, UFRJ, in addition to functional assessment scales, to monitor the rehabilitation of 25 patients with lesions of the brachial plexus, the bundle of nerves that lead from the spinal cord and innervates the arms. These injuries are common in motorcyclists, especially motorcycle couriers who damage some or all of the nerves, losing part or all of the sensitivity and movement of one of their arms.
It has already been observed that some patients regain some movement after nerve restoration surgery and intense physical therapy. But researchers want to know how functional networks adapt to the rearrangement of nerves and whether, using this new information, they could improve the rehabilitation process.
Doctors, nurses and physical therapists associated with the project are collaborating with neuroscientists to collect patients’ functional measurements and feed a large database developed by another NeuroMat group, coordinated by computer scientist Kelly Braghetto, of IME-USP.
This database is now of central importance to NeuroMat. The labeled and organized data can be shared with other researchers and submitted to original proposals for analysis. The NeuroMat researchers hope to apply the same approach at Lucy Montoro Rehabilitation Network clinics, directed by physiatrist Linamara Battistella, of USP, where patients with brain injuries caused by strokes are participating in clinical studies.
One project compares the recovery of arm movements in patients treated with two different rehabilitation techniques. Statisticians Jesús García and Verónica González-López, both from Unicamp and associated with NeuroMat, are working together to eliminate redundancies in the clinical assessment questionnaire and optimize the scales used to assess patient motor ability.
The evolution of this capacity is also being monitored with measurements of brain activity using electroencephalography and transcranial magnetic stimulation. “The measurements allow us to better understand the functional changes that occur in the nervous system and to correlate these changes with the clinical improvement of patients who have had a stroke,” explains neurologist Marcel Simis, one of the researchers participating in the project.
Project
Research, Innovation and Dissemination Center in Neuromathematics – NeuroMat (No. 2013/07699–0); Grant Mechanism Research, Innovation and Dissemination Center (RIDC); Principal Investigator Jefferson Antonio Galves – IME/USP; Investment R$11,755,168.93 (FAPESP) for the entire RIDC.
Scientific articles
FRAIMAN, D. et al. Biological motion coding in the brain: analysis of visually driven EEG functional networks. PLOS One. v. 9, n. 1. Jan. 2014.
GALVES, A. and LÖCHERBACH, E. Infinite systems of interacting chains with memory of variable length—A stochastic model for biological neural nets. Journal of Statistical Physics. v. 151, n. 5. Jun. 2013.