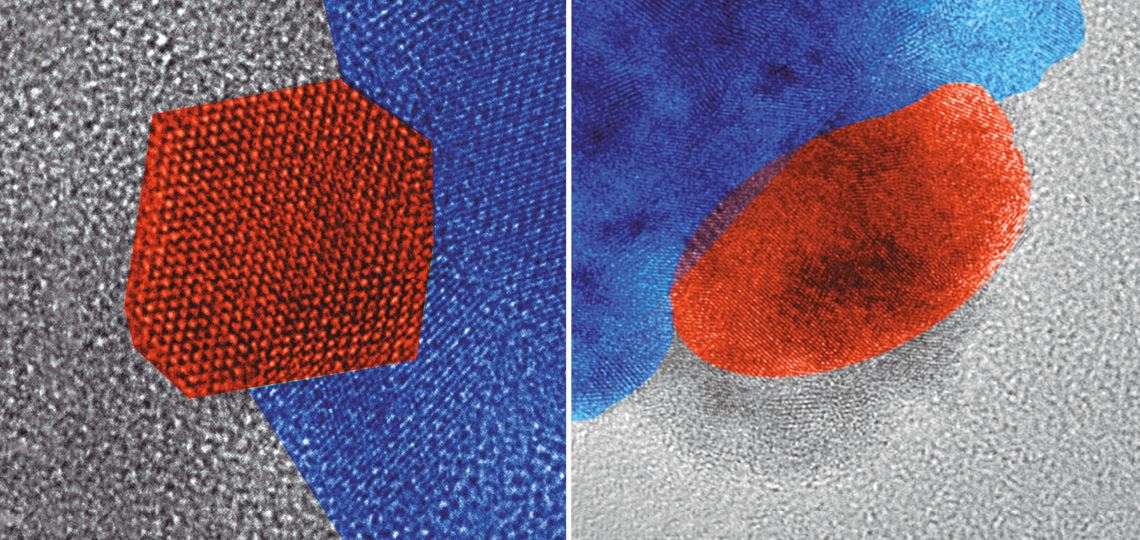
When subjected to carbon monoxide treatment, copper nanoparticles (in orange) flatten against a support (blue), increasing the contact surface area. The transformation from three-dimensional (angular shape) into two-dimensional (flat) could be useful in experiments and technologies involving catalyst materials, in which the interaction between nanoparticles and substrates can accelerate chemical reactions. “Usually, hydrogen is used to activate catalysts before starting the reaction,” say pharmacist Fernanda Poletto and physicist Fabiano Bernardi, who discovered the surprising effect.
Image submitted by Fernanda Poletto, a professor at the Institute of Chemistry of the Federal University of Rio Grande do Sul (UFRGS)
Republish