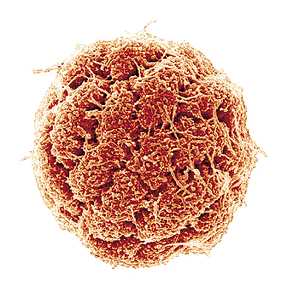
ULISSES LINS/UFRJA magnetic bacterium from Araruama shows its cells under the electronic microscope scannerULISSES LINS/UFRJ
It is estimated that one third of the live beings on this Earth are microscopic organisms that take up all the outer and inner spaces of other organisms. Most of these are single-cell bacteria. But this is not always the case. The team working under Ulysses Lins, from the Federal University of Rio de Janeiro (UFRJ), recently described in the International Journal of Systematic and Evolutionary Microbiology a bacterium discovered in the Araruama lagoon, on the coast of the state of Rio de Janeiro. The team named their recent discovery Candidatus Magnetoglobus multicellularis. As the name indicates, these are round, multi-cell bacteria with magnetic properties. “This is not the first multi-cell bacteria, nor the only one,” explains Lins. “The novelty is that it does not go through a single-cell stage during its life cycle; it is unable to respond to the magnetic field when the cells separate from the microorganism and its motility is characteristic of a multiple set of cells and not of individual cells.”
The high salt content of the Araruama lagoon’s waters, one of the largest salty coastal lagoons in the world, is a challenge to any form of life. Nonetheless, bacteria are able to colonize these hostile waters and this is why it is fertile ground for research for microbiologists such as Lins. The group collects samples of the water, which are stored in sealed containers until visible layers are formed, with water at the top and thicker sediment at the bottom. From time to time, the team places a drop of the sediment on a slide and examines this under the microscope with a magnet next to it. The magnetotatic bacteria have tiny metallic particles inside; these particles are aligned and function as if they were internal magnets. This compass helps them, for example, to distinguish where the deeper layers with less oxygen are, their favorite environment. In the Southern Hemisphere, the bacteria always swim towards the south, so a magnet with the north end pointed towards the slide attracts the bacteria, making them visible under the microscope. In order to study the bacteria in detail, it is necessary to purify the sample. The researchers do this by attaching a small magnet to the side of a plastic tube that contains sediment, and by drying the equipment with sterilized water from the lagoon. After this process, only the magnetotatic bacteria remain in the tube, which allows the microbiologists to observe them with an electronic microscope and film them under the optical microscope.
Lins and his team, which includes other researchers from the Federal University of Rio de Janeiro (UFRJ), from Rio de Janeiro’s Brazilian Physical Research Center (Centro Brasileiro de Pesquisas Físicas) and from the Institute of Chemistry at the University of São Paulo/ USP, used this technique to describe in detail the characteristics of Candidatus Magnetoglobus multicellularis. They found a unique set of cell architecture, life cycle, magnetic properties, coordinated movements and type of habitat. In addition, genetic data confirmed that the bacteria must be classified as a new species. This has not happened yet, as the word Candidatus, placed in front of the proposed name, indicates. For the species to be validated, it is necessary to isolate it from its environment and maintain it in a culture. This is harder than it may seem, and has not been achieved yet in the case of the Candidatus Magnetoglobus multicellularis. “We probably don’t know how to cultivate it because we are unfamiliar with the metabolism of these bacteria and therefore we don’t know very much about their nutrition requirements,” explains the researcher from UFRJ.
Disclosed details
This bacteria from Rio de Janeiro is shaped like a hollow sphere of about 4 thousandths of a millimeter, and is comprised of several coil-shaped cells. The number of cells ranges from ten to forty, and all of the cells have contact with the outside environment and the internal cavity. These cells are covered in flagella, tiny tails that work like oars, which moves the organism forward. If each cell were an independent bacterium, then the flagella would move on their own and the group of cells would remain still or would move at random. This is not the case of the bacteria from Araruama lagoon. Each cell has some 30 flagella, totaling up to 1.200 per bacteria. All the flagella move in sync. Lins believes that the secret lies in special connections that link the cells, allowing them to communicate somehow. This is quite astonishing, because this kind of structure has never been described before in bacteria – only in more complex organisms.
Each one of the cells contains from 60 to 100 magnetosomes, slightly oval spheres measuring less than one millimeter divided by 10 thousand. These spheres are responsible for guiding the bacteria – the so-called internal compass. During the reproduction phase, each cell multiplies or increases its internal particles including the magnetosomes, and then splits into two. The sphere then has double the number of cells and forms a constriction that leads to two identical spheres; each cell has one half of the cells of the source bacteria. In the course of this process, the internal cavity does not come into contact with the outer environment and, unlike other multi-cell bacteria, the Candidatus Magnetoglobus multicellularis never goes through the one-cell stage. As in movement, reproduction requires cell coordination and this is why this discovery in the Araruama lagoon is so special. If a cell is isolated from the organism, it dies, which proves the need for a full set for it to live. To date, these characteristics have been considered to be unique to eukaryotes, a kind of multi-cell organism that is part of all live beings visible to the naked eye.
How bacteria move
Candidatus Magnetoglobus multicellularis so far has shown that it has four different ways of moving around. In a uniform magnetic field, the researchers observed what they referred to as the free movement, where the bacteria swim in a straight or coiled line. When they are at the edge of the drop in which they are being observed under the microscope, they rotate around their own axis. In the interface between water and air, they “walk” (“walk” is the term used by Lins’ team) on complex paths, always facing forward. The most peculiar movement is when they run away; this movement is also referred to as ping-pong: the bacteria recoil rapidly and then go in another direction.
It is tempting to imagine that this astonishing discovery might reveal how the transition between primitive bacteria and more complex organisms occurred at the beginning of evolution. This may be tempting, but not necessarily right, warns Lins. “The belief is that multi-cell characteristics have surfaced independently a number of times in the course of the evolution of life, in bacteria or in non-bacterial organisms. Multi-cell bacteria are one of these incursions”, the microbiologist explains. He does not discard the hypothesis that multi-cell bacteria are similar to the bacteria that began the long evolutionary path resulting in insects, vertebrates, plants and other visible organisms. But this was not necessarily so. “It is quite possible to imagine that a non-bacteria cell associated itself with others of the same kind to form a plural-cell organism, independently from the bacteria.”
Republish