More than 20 years of research undertaken at the University of São Paulo’s São Carlos Institute of Physics (IFSC-USP), in the interior of São Paulo State, have resulted in a new therapeutic protocol for a specific type of skin cancer, and an innovative apparatus. Both can be used to diagnose and treat tumor lesions, and the innovation is about to be rolled out to the Brazilian public on recommendation of the Brazilian Commission for the Incorporation of Technologies (CONITEC) to the Brazilian Unified Health System (SUS). CONITEC is an advisory body to the Brazilian Ministry of Health on matters relating to the adoption, exclusion, or alteration of healthcare technologies by SUS.
In July this year, after evaluating scientific safety and efficacy evidence, along with economic feasibility studies, CONITEC recommended that SUS incorporate photodynamic therapy (PDT) for basal cell carcinoma, one of the most common types of skin cancer in Brazil.
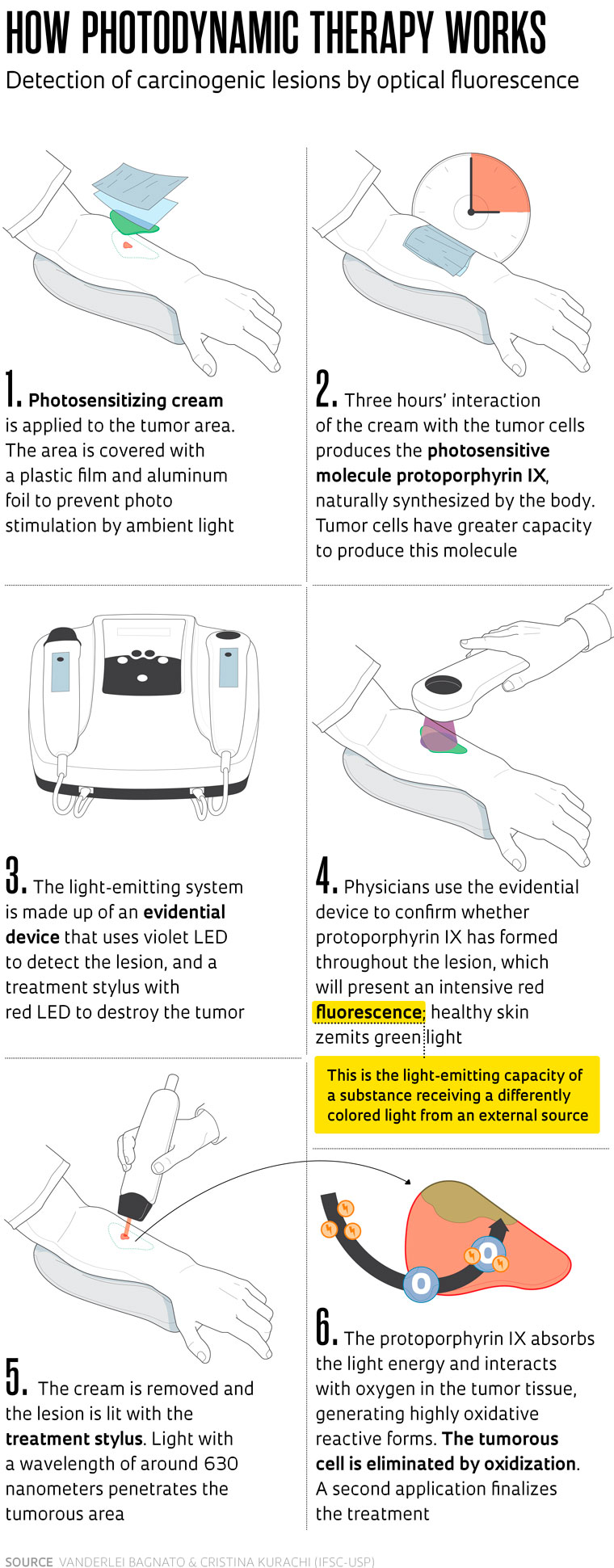
The new treatment involves destruction of the tumor through intensive light radiation in a wavelength that activates a photosensitive agent produced by the tumor cells after local application of a cream. Once activated this agent produces free radicals that destroy the cells containing it. Prior to the radiation, the photosensitive agent enables identification of the tumor area—once illuminated by blue light, the agent glows red (see infographic on page 68).
The CONITEC endorsement was published in Diário Oficial da União (Official federal gazette) on September 5. The Health Ministry was given 180 days from that date to implement this offer across the SUS. “The incorporation of this technology is an incentive for universities to keep investing in research, essential for promoting scientific and technological developments in healthcare,” comments pharmacist Daniela Oliveira de Melo, coordinator of the Healthcare Technology Evaluation Center at the Federal University of São Paulo (UNIFESP) and professor of the institution’s pharmacy course. The faculty collaborated with the Brazilian Ministry of Health in their analysis.
Physicist Vanderlei Bagnato, the project lead, states that he started working with photodynamic therapy around 1997, when he learned of PDT in cancer treatment during a conference in the United States. “I was fascinated, and started to apply the concept in clinical practice in partnership with the Amaral Carvalho Hospital in the town of Jaú [São Paulo state],” he says. Bagnato coordinates the Optics Group at IFSC-USP, headquarters of the Optics and Photonics Research Center (CEPOF), one of the Research, Innovation, and Dissemination Centers (RIDC) supported by FAPESP.
The physicist initially used laser to treat tumors in the buccal cavity and internal organs such as the esophagus and bladder. The photosensitizer drug, capable of absorbing certain beams of light and eradicating diseased cells, was systemically administrated intravenously. “Despite the promising results in bladder and esophagus cancer treatment, we started to focus on skin cancer in 2005, using a topical medication [applied to the skin].”
There were two key motives for shifting the focus: the first was the significant number of skin cancer cases in Brazil that could respond quickly to the treatment, and the second involved the therapy itself. Since the treatment was topical the results were more easily visible, which helped in developing the technique.
The Brazilian Cancer Institute (INCA) estimates that Brazil will report 704,000 new cases of cancer per year to 2025. The most frequent is caused by nonmelanoma skin tumors, accounting for 31% of cases among all tumor types—some 220,000 new cases annually. Basal cell carcinoma, for which the photodynamic therapy was recommended, is the most common nonmelanoma skin cancer subtype, corresponding to 80% of the total.
According to dermatologist Mário Yoshiaki Enokihara, coordinator of the Advanced Surgical Dermatology Specialization course at UNIFESP, basal cell carcinoma presents a favorable prognosis provided it is treated early, when the lesion is incipient. It was exactly for the treatment of surface tumors infiltrating up to 2 millimeters (mm) deep that the São Paulo researchers created the protocol, using light-emitting diodes (LED). The laser, which penetrates deeper into the skin, was used in studies into the treatment of internal organs.
“Not all patients treated in public service are diagnosed early, when tumors are still superficial,” says Enokihara. Photodynamic therapy is also recommended in specific cases where invasive treatment is contraindicated, whether due to clinical patient restrictions such as immunosuppression or poor lesion-healing capacity, or where there is a high risk of disfigurement of the area operated, with functional compromise. The gold standard for treatment is surgical tumor removal, with a healing rate of up to 98%.
The company MM Optics, an IFSC spin-off founded in 1998 and supported by FAPESP, was tasked with creating the new PDT apparatus, with LED light, under the guidance of the institute’s Optics Group. The equipment, 100% Brazilian, was backed by the Brazilian Funding Authority for Studies and Projects (FINEP) and the Brazilian Agency for Industrial Research and Innovation (EMBRAPII) (see Pesquisa FAPESP issue nº 253). It received formal approval from the Brazilian Health Regulatory Agency (ANVISA) in 2014.
According to electrical engineer Anderson Luís Zanchin, industrial and engineering director at MM Optics, what differentiates this apparatus from others used for PDT by private clinics is a dual system of photo detection—emission of light to view the tumor—and an application stylus. Rival equipment only has the latter. The use of PDT for nonmelanoma skin cancer was approved in Brazil in 2006.
Multicentric study
The company has manufactured some 200 devices, currently used by research centers and private medical services. “Brazil will be the first country ever to roll out photodynamic therapy across the public health system,” says researcher Cristina Kurachi, also involved in PDT work at IFSC since the end of the 1990s.
In recommending that the technology be incorporated into SUS, CONITEC considered the availability of trained professionals and facilities across dozens of public healthcare units. Kurachi states that this structure is the result of the TFD Brasil Program, coordinated by IFSC with the backing of the Brazilian Development Bank (BNDES).
Commenced in 2012, the program was devised to implement PDT in national centers. Over the five years of the project, professionals from 72 treatment centers around the country were trained in the new technology. “It was the world’s biggest multicentric clinical skin cancer trial,” says Bagnato. An article with the results was published in the journal Cancer Control in 2019.
Among other agencies, the trial involved INCA, which created a specific outpatient center to treat skin cancer using photodynamic therapy in 2015. “The procedure spares patients with superficial lesions from surgery,” points out the institute’s dermatology section chief Dolival Lobão. He goes on to explain that, in addition to obviating the need for anesthesia and hospital admission, PDT leaves no scars in the treated area. On average the service attends to three patients per week.
Low-cost photosensitizer
The INCA partnership with USP is currently ongoing. IFSC provides the equipment and cream used in the therapy (pharmaceutically manipulated at the university) free of charge, with INCA referring the clinical results to researchers. Private clinics rely on an imported product that costs around R$1,000 for a 2-gram tube, enough for an average of four applications. Manipulation of the drug at USP was funded by BNDES and EMBRAPII, enabling its free provision to INCA. A more economical solution may follow soon: the company Emipharma, linked to MM Optics, is being set up for Brazilian production of the medication.
Obtaining CONITEC recommendation has been a long process. The first application for incorporation of the therapy was lodged in November 2018, and after a year of analysis it was rejected. The commission analyzing the application considered the scientific evidence to be tenuous, primarily in terms of its efficacy compared to surgery. “We persisted with refinement of the technique and raised the tumor elimination rate to 95%,” recalls Bagnato. “We applied safety margins to ensure the removal of small cells at the edges, outside the main tumor mass.”
In August 2022 a new application was submitted and the preliminary opinion, issued in March 2023—before the public consultation stage—was still unfavorable. Misgivings persisted over the technique, chiefly in terms of cost effectiveness. “After the public consultation, CONITEC held another meeting and we were able to provide full clarification,” says Kurachi. The final opinion, this time favorable, was issued in July.
Dermatologist Enokihara, of UNIFESP, still holds reservations over the implementation of PDT in cancer treatment. His primary concern is the correct indication of photodynamic therapy and training for professionals who will apply it. “Among basal cell carcinoma cases alone there are several types, some more aggressive than others. Diagnosis needs to be spot-on,” he warns.
Dermatologist Maria Claudia Almeida Issa, of Fluminense Federal University (UFF), somewhat agrees. She has been studying PDT for more than 15 years and has seen the technique used improperly in certain cases. For example, she does not recommend its application for basal cell carcinoma in skin areas with a high risk of recurrence. “In the area of the nose and close to the eyes, even the in-situ [noninvasive] carcinoma is aggressive. In such cases PDT is not recommended due to the high relapse rate,” she says. The specialist highlights that PDT has “an excellent healing rate for premalignant actinic keratoses (skin lesions) on the face.
Author of a review article on the use of PDT in skin treatment published in the journal Anais Brasileiros de Dermatologia in 2010, and a participant in the multicentric study promoted by the São Carlos group, Issa regards incorporation of the treatment into the public system as positive. “I am happy that this therapy has been approved for implementation across SUS. Now its application will be standardized.”
Projects
1. CEPOF – Optics and Photonics Research Center (nº 13/07276-1); Grant Mechanism Research, Innovation, and Dissemination Centers (RIDC); Principal Investigator Vanderlei Salvador Bagnato (USP); Investment R$44,106,793.11.
2. Precision optical microscope with synthetic granite-based structure (nº 99/06402-4); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Fernando de Moraes Mendonça Ribeiro (MM Optics); Investment R$45,340.92.
Scientific articles
BUZZÁH. H. et al. Overall results for a National Program of Photodynamic Therapy for basal cell carcinoma: A multicenter clinical study to bring new techniques to social health care. Cancer Control. Vol. 26, no. 1, pp. 1–12. 2019.
ISSA, M. C. A. & MANELA-AZULAY, M. Terapia fotodinâmica: Revisão da literatura e documentação iconográfica. Anais Brasileiro de Dermatologia. Vol. 85. Aug. 2010
Book
BAGNATO, V. S. & REQUENA, M. B. (org). Terapia fotodinâmica dermatológica: Programa TFD Brasil. IFSC-USP. Even3 Publicações Editora, 2023.
Republish