Brazilian scientists observed the formation and rupture of zirconium dioxide (ZrO2) ionic wires on an atomic scale using a high-resolution electron microscope, in a vacuum, at room temperature. The material has a wide range of applications in catalysts, fuel cells, water purifiers, and lithium-ion batteries. In the final stages of rupture, empty spaces known as voids formed spontaneously between the oxygen atoms in the nanometric wires. The voids increased the length and malleability of the nanowires, which proved to be highly stable—a rare property (Physical Review Letters, July 18). The researchers involved in the study—from the federal universities of ABC (UFABC), São Carlos (UFSCar), and Rio de Janeiro (UFRJ), the Brazilian Center for Research in Energy and Materials (CNPEM), and Harvard University, USA—say the results show the ionic compound can be added to the list of materials that can form monatomic wires and help us understand the mechanical properties of ceramic materials at the nanoscale. In 2002, researchers from the group described how nanowires made of gold rupture.
RepublishNanotechnology
Rupture-resistant nanowires
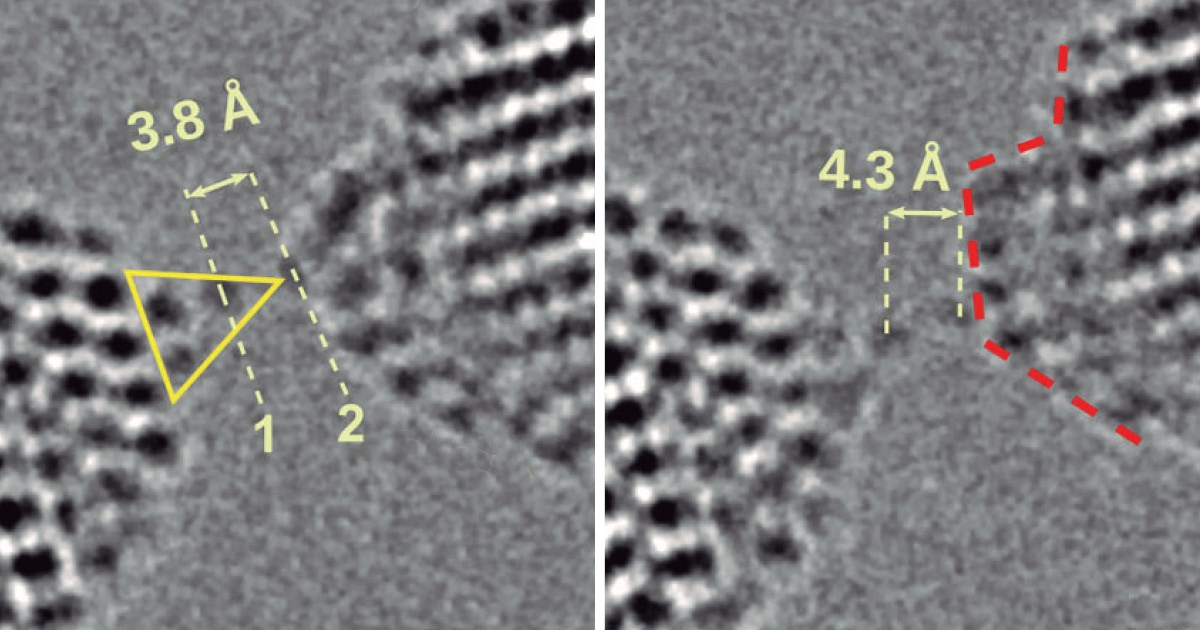
Images showing the increasing distance between atoms and emergence of empty spaces
FOCASSIO, B. et al. Physical Review Letters. 2022