 ZÉ VICENTEIn an experiment thought to be impossible until last year, a team coordinated by physicist Roberto Sierra, of the Federal University of the ABC (UFABC), determined how much energy an atomic nucleus can gain or lose when it is hit by a radio wave pulse. Most researchers were convinced that the nucleus’ behavior would be unpredictable. It was believed that we would never know the probability that the nucleus would absorb the wave’s energy, and thus become hotter, or conversely transmit some of its energy to the waves and thus become cooler.
ZÉ VICENTEIn an experiment thought to be impossible until last year, a team coordinated by physicist Roberto Sierra, of the Federal University of the ABC (UFABC), determined how much energy an atomic nucleus can gain or lose when it is hit by a radio wave pulse. Most researchers were convinced that the nucleus’ behavior would be unpredictable. It was believed that we would never know the probability that the nucleus would absorb the wave’s energy, and thus become hotter, or conversely transmit some of its energy to the waves and thus become cooler.
The new experiments carried out at the Brazilian Center for Physics Research (CBPF), in Rio de Janeiro, demonstrate that this energy exchange obeys physical laws never before tested on the subatomic level. These laws could help us better understand chemical reactions like plant photosynthesis and determine how much energy quantum computers will need to operate. “This is the first experiment in a new area of physics, called quantum thermodynamics,” says Serra.
Quantum computers are expected to exponentially surpass the calculation power of conventional computers by taking advantage of the laws of quantum mechanics. But how much power will this new type of computing need in practice? How much heat will these machines produce? Will they need refrigeration? One of the goals of quantum thermodynamics is to answer these questions.
Similar questions abounded during the Industrial Revolution, in the nineteenth century. In order for steam engines to reach their maximum efficiency, what is the minimum amount of coal that furnaces require and at what temperature should boilers be maintained? Scientists of the era realized that both heat and the ability of machines to work are different forms of the same physical quantity, energy, which is never created from nothing nor destroyed, only transformed. When investigating the conversion of one form of energy into another, they discovered the laws of classical thermodynamics.
According to these laws, energy flows spontaneously from a hotter body to a cooler one. And a machine, even if ideal, can only convert part of the available energy in the form of heat into energy able to perform mechanical movements, that is, to perform what is known in physics as work. “Thermodynamics imposes limits on any technology,” says Serra.
Victorian engineers solved their problems, but at the expense of a little trick. Their calculations only worked when machines were assumed to be insulated from the rest of the world, exchanging little heat with the environment. The processes also had to be slow. But these approximations do not apply to most situations that occur in nature—for example, in many chemical reactions. When an object cannot be thermally isolated from its environment for a long time, its temperature rises and falls in a seemingly unpredictable way, contrary to what occurs in isolated systems, where everything tends towards equilibrium.
It was only in 1997 that the physical chemist Christopher Jarzynski discovered a mathematical expression for calculating the variations in energy and mechanical work when out of equilibrium. “Jarzynski’s equation and other theorems on fluctuations allowed chemists to measure, in a laboratory, the variation in energy of a molecule before and after a reaction,” explains Serra.
Jarzynski, in collaboration with a team in California, confirmed his equation in 2005, observing the mechanical work of an RNA molecule stretched and compressed like a spring. Serra notes, however, that despite being microscopic, the movement of the RNA molecule was large enough to be calculated using the famous formula derived from the laws of Newtonian mechanics: “Work is equal to force times displacement.”
 The equations of thermodynamics, whether in equilibrium or not, were derived using Newtonian mechanics. But Newton’s laws lose their meaning for various processes taking place in molecules and for anything occurring within atoms because forces and displacements cannot be measured precisely. On these scales, the laws of quantum mechanics apply. Serra wanted to know if equations like Jarzinsky’s were still applicable at the subatomic scale. This knowledge will help us understand chemical reactions such as photosynthesis. In photosynthesis, molecules in the cells of leaves act like quantum machines that absorb energy from light particles and store it in the form of sugar molecules. “The process is very efficient, and generates almost no heat,” says Serra. “Studies suggest that it is a quantum process.”
The equations of thermodynamics, whether in equilibrium or not, were derived using Newtonian mechanics. But Newton’s laws lose their meaning for various processes taking place in molecules and for anything occurring within atoms because forces and displacements cannot be measured precisely. On these scales, the laws of quantum mechanics apply. Serra wanted to know if equations like Jarzinsky’s were still applicable at the subatomic scale. This knowledge will help us understand chemical reactions such as photosynthesis. In photosynthesis, molecules in the cells of leaves act like quantum machines that absorb energy from light particles and store it in the form of sugar molecules. “The process is very efficient, and generates almost no heat,” says Serra. “Studies suggest that it is a quantum process.”
Serra, his students and colleagues at UFABC tried for some time to study quantum thermodynamics in the laboratory, along with the team of physicists Alexandre Souza, Ruben Auccauise, Roberto Sarthour and Ivan Oliveira, who work with nuclear magnetic resonance techniques at CBPF. The partnership between the two groups has already resulted in several discoveries (see Pesquisa FAPESP Issue No. 193).
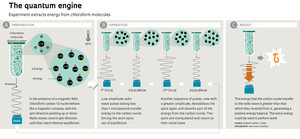
A small test tube containing a solution of pure chloroform diluted in water is located at the heart of the equipment in the CBPF laboratory. Each of the approximately 1 trillion chloroform molecules in the solution contains a carbon-13 atom. The nucleus of this type of carbon has a quantum property called spin, which is represented like the arrow of the needle of a magnetic compass. Under a strong magnetic field parallel to the tube, pointing upwards, the spin arrows of the carbon atoms tend to align with the field, half of them pointing down and half up. The magnetic field also causes the atoms with spins pointing downwards to have more energy than those with spins facing upwards.
Physicists manipulate the spin directions using electromagnetic fields that oscillate at a frequency of 125 megahertz (the equipment must be electro-magnetically insulated so that it is not influenced by FM radio stations that transmit at that frequency). This is done using wave pulses that last no more than a few microseconds. The experiment takes place so quickly that it is as if each carbon atom in the test tube were isolated from the rest of the universe, for a brief moment, and subjected to a temperature close to absolute zero ( 273º Celsius). The researchers are able to increase or decrease the energy difference between the atoms with up and down spin by reducing or increasing the amplitude of the radio waves. When the amplitude changes very quickly, the carbon atoms are no longer thermally isolated and begin to both absorb energy from radio waves—which is when the waves do work on the atoms—and transmit part of their energy to the waves, performing work on them. “This is very difficult to measure because carbon atoms with spin can exchange energy in one of four ways, all happening at once, in a probabilistic manner,” says Serra. “I know of a group in Germany that tried to carry out the same experiment for five years without success.”
What hindered the success of the German group, according to Serra, was the fact that the physicists tried to directly measure how many times energy was emitted or absorbed by the atoms. “The cumulative error in these measurements was so great that, in the end, they were unable to determine anything,” he explains.
Intelligent measurement
The solution came earlier for Serra, in February 2013, when the physicist Mauro Paternostro, of Queen’s University in Belfast, Ireland, gave a seminar at UFABC on unpublished proposals to observe the work produced by light particles indirectly. Soon Paternostro, currently a visiting professor at UFABC, and Laura Mazzola, his colleague in Belfast, began discussing with Serra, Auccauise and UFABC doctoral student Tiago Batalhão how to adapt these techniques to observe the work due to carbon atom spin indirectly. With John Good, of the University of Oxford, England, the team discovered a clever way to use the spin of the hydrogen nuclei in chloroform molecules to indicate what happens to the spin of carbon atoms while performing work, without interfering in the process.
The precision of the experiment was sufficient to record temperature variations in carbon spin on the order of billionths of a degree and verify that the Jarzinsky equation is valid on the subatomic scale. Another interesting result: the carbon atoms with spin had a greater tendency to extract energy from the radio waves when the amplitude of the wave pulse was smaller. The opposite happened when the wave amplitude was increased: the atoms with spin tended to transfer energy to the waves—or in other words, perform work on the waves.
“We could exploit this difference to create a quantum heat engine,” says Serra. The engine would work by alternating low and high amplitude pulses between two states in thermal equilibrium, each with a different temperature (see infographic). The engine would work in a manner similar to that of a combustion engine, which performs mechanical work with some of the chemical energy transformed into heat due to the explosion of the fuel.
The spin engine would not be very useful: the work produced would supply such a small amount of energy to the radio waves that it would only be sufficient to alter the spin of an atomic nucleus. Serra is more interested in measuring how much energy it uses and how much heat it dissipates during operation.
“The technique applied in this experiment has great potential,” says physicist Lucas Céleri, of the Federal University of Goiás, who intends to observe the thermodynamics of a single photon together with physicists Paulo Souto Ribeiro and Stephen Walborn, of the Federal University of Rio de Janeiro, at the beginning of 2015. “Experimental advances are very rare in quantum thermodynamics, because of the need to control the quantum system and isolate it from the environment.”
Project
National Institute of Quantum Information Science and Technology (nº 2008/57856-6); Grant Mechanism Thematic Project; Principal investigator Amir Caldeira (Unicamp); Investment R$1,384,811.24 (FAPESP) and R$5,700,000.00 (CNPq).
Scientific article
BATALHÃO, T. B. et al. Experimental reconstruction of work distribution and study of fluctuation relations in a closed quantum system. Physical Review Letters. 113 (14). 3 Oct. 2014.