 Eduardo CesarIn the search for more efficient micro organisms for the production of ethanol, two Brazilian researchers used distinct methods to develop two new yeast strains of the Saccharomyce cerevisiae species, able to produce higher quantities of the substance. Yeasts – tiny, microscopic fungi – play a crucial role in the transformation of sugar into alcohol during the fermentation process in sugar mills. The group headed by professor Boris Ugarte Stambuk, from the Federal University of Santa Catarina/UFSC, resorted to genome engineering, while the researchers coordinated by professor Cecília Laluce, from Paulista State University/Unesp in Araraquara, São Paulo State, resorted to classic genetics to obtain a hybrid from selected yeasts. “We intervened in the genome of the Saccharomyces to modify how it acts on the fermentation and thus we were able to optimize the process”, says Stambuk, of UFSC’s Biochemistry Department at the Center of Biology Sciences. Stambuk also coordinates a research group at the institution, created in 1997, that conducts research studies on molecular biology and yeast biotechnology. “We were able to obtain 10% to 15% more ethanol with the same quantity of sucrose”.
Eduardo CesarIn the search for more efficient micro organisms for the production of ethanol, two Brazilian researchers used distinct methods to develop two new yeast strains of the Saccharomyce cerevisiae species, able to produce higher quantities of the substance. Yeasts – tiny, microscopic fungi – play a crucial role in the transformation of sugar into alcohol during the fermentation process in sugar mills. The group headed by professor Boris Ugarte Stambuk, from the Federal University of Santa Catarina/UFSC, resorted to genome engineering, while the researchers coordinated by professor Cecília Laluce, from Paulista State University/Unesp in Araraquara, São Paulo State, resorted to classic genetics to obtain a hybrid from selected yeasts. “We intervened in the genome of the Saccharomyces to modify how it acts on the fermentation and thus we were able to optimize the process”, says Stambuk, of UFSC’s Biochemistry Department at the Center of Biology Sciences. Stambuk also coordinates a research group at the institution, created in 1997, that conducts research studies on molecular biology and yeast biotechnology. “We were able to obtain 10% to 15% more ethanol with the same quantity of sucrose”.
The strategy was to modify how the Sacaccharomyce produce the invertase enzyme, responsible for speeding up the hydrolysis of sucrose carbohydrates, transforming them into glucose and fructose. This reaction, which occurs outside the yeast cell, is called extra cellular hydrolysis. Altering the invertase by modifying the specific gene for this enzyme, the sugar is transported and fermented directly inside the Saccharomyces. “In my opinion, extra cellular hydrolysis is an inefficient system because it allows other yeasts and bacteria found in the fermentation vats to use the glucose and the fructose”, says Stambuk. Stambuk is also an accredited advisor of the University of São Paulo/USP’s Post-Graduate Program in Biotechnology. When these contaminating microorganisms ferment, they produce organic acids that result in ethanol production losses.
The next step of the research project, which included the participation of researchers from USP’s e Chemistry Institute and from the Escola Superior de Agricultura Luiz de Queiroz de Piracicaba, School of Agronomy, will be to test the genetically modified yeast at the Usina Cerradinho mill in the city of Catanduva, São Paulo State, to evaluate how it behaves in an industrial environment. The study obtained funding from FAPESP, by means of a Theme Project coordinated by professor Pedro Soares de Araújo, from USP’s Chemistry Institute and from the Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq agency. These institutions approved the proposal submitted by Stambuk in partnership with Fermentec, a consulting firm specialized in alcohol fermentation, which had announced a bid for Technological Development and Innovation. Stambuk says that his interest in how to capture sugar directly through yeast began in 1997. Some studies that had already raised this possibility had been conducted by Spanish and Australian researchers in the 1980s, but these studies did not progress. Prior to beginning the project that resulted in the genetically modified yeast, the researcher had been advisor to two students in the master’s program. These students had discovered the gene responsible for the direct capture of the sugar. “It had already been described in literature that this was possible with the fermentation of maltose, another kind of sugar that the Saccharomyce ferments efficiently”, says Stambuk. “The yeast throws the maltose into the cell and ferments the sugar from the inside”.
Ever since the genetically modified yeast project began in 2005, several strategies have been tested to make the yeast stop producing extra cellular invertase and start carrying the sugar into the inner part of the cell, where the hydrolysis of the sucrose occurs. One of the attempts was successful and resulted in the filing of a patent in April this year, in partnership with Fermentec. This project has also resulted in several master’s dissertations and doctorate theses in progress, under the advice of Stambuk and presented at UFSC and USP. Professor Ana Clara Guerrini Schenberg, from USP’s Biomedical Institute, who has conducted research studies on yeasts since the 1970s, has emphasized that this new strategy is innovative. “This is a new way of making yeast produce alcohol”, said Ana Clara, who was on the evaluation committee of one of Stambuk’s students. The researcher, who is conducting a number of projects in this field, including a project on the genome sequencing of industrial yeasts, explains that the genetic modifications become stable inside the yeast because they occurred in the chromosomes themselves. “Many modifications are made with plasmids for yeasts, genetic material which is also found in bacteria. However, this does not work in the industrial field, because these molecules are unstable”.

EDUARDO CESARFormation of yeast colonies of the Saccharomyces speciesEDUARDO CESAR
Resistance to heat
The other yeast strain that proved to be an excellent producer of alcohol when tested in the lab has another characteristic that makes it appropriate for the conditions that exist in industrial processes. This yeast strain is resistant to high temperatures. “Commercial yeasts for the production of ethanol ferment well in temperatures from 30ºC e 34ºC; the yeast that we developed ferments in temperatures from 37ºC to 38ºC with little cell mortality”, says Cecília. As it is very difficult to control the temperature of the fermentation process in the summer, any temperature above 36ºC immediately increases the toxicity of the alcohol in the vats, which results in the death of the ethanol-producing yeasts. Another innovation of this new strain, which is also the object of a patent request filed by the Agência Unesp de Inovação agency in September 2008, is that it ferments quickly. “It makes the total conversion of sugar in up to three hours, while in the traditional process, fermentation takes from six to twelve hours”, says the researcher, who has been studying yeasts since the 1980s. This is an advantage because the longer the fermentation time, the stronger the effects of the contaminant microorganisms and of other aggressive factors, such as high temperatures and nutritional deficiency, in the fermentation process. The new strain is also resistant to high quantities of ethanol and to the acidity found in successive fermentation cycles.
In order to achieve this result, the researchers selected various strains of the S. cerevisiae found at sugar mills; these strains had to have specific characteristics, such as tolerance to heat and fast consumption of sugar for the production of ethanol. After various tests and combinations, the researchers obtained a hybrid yeast which was given genetic markers that allow monitoring to be conducted during the entire alcoholic fermentation process. “The markets show the proportion of this yeast in relation to the contaminant microorganisms found in fermentation vats”, says Cecília. In addition, it’s possible to observe if the fermentation cells are going through modifications during harvesting, if the yeast is dominated by wild yeasts and even if it disappears from the process, because it was dominated by its competitors. At present, all of this is being done by means of molecular biology techniques which need specialized consultants.
“At the sugar mill, the same yeast is used in various fermentation cycles during the entire harvest, which lasts up to seven months”, says researcher Karen de Oliveira, who worked with hybrid yeast during her doctorate studies under Cecília. Karen concluded her doctorate studies in 2008. “In some cases, three different species of Saccharomyce are used at the mills at the beginning of the harvest and after a month, there are no yeasts left”, she reports. The yeasts found in the environment or in the raw materials invade the ethanol production process and begin to multiply. “But the yeasts in the environment only have to feed themselves – they don’t have to produce alcohol”, says Karen, who is currently doing research on the behavior of yeasts during fermentation of sugar cane bagasse hydrolisates as part of her post-doctorate studies. The project, coordinated by professor Cecilia Laluce, and includes researchers Sandra Sponchiado and Eduardo Cilli, is part of the Programa FAPESP de Pesquisa em Bioenergia/Bioen bioenergy research program.
“Controlling the stability of yeasts during the harvest is crucial to ensure the continuity of the successive fermentation cycles”, says Cecilia. The yeast dies when the fermentation starts getting intoxicated because of excess alcohol being produced or as a result of fermentation stress conditions. This can result in the need to start the entire process over again. “The collapse of a process, with complete stoppage and re-starting means huge losses for the sugar mills”, she emphasizes. The next step of the project, which is already being negotiated with the Centro de Tecnologia Canavieira/CTC Sugar Cane Technology Center, is to test the yeast in an industrial process.
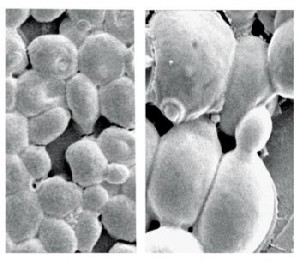
Chemistry institute /unesp araraquaraYeast cells seen under electronic microscopeChemistry institute /unesp araraquara
Induced mutation
The Saccharomyce cerevisiae yeast has stress-support mechanisms, as demonstrated by the lab studies conducted by professor Sandra Regina Ceccato Antonini, of the Centro de Ciências Agrárias, Agronomy Studies Center at the Federal University of São Carlos/UFSCfar), Araras campus. Some factors, such as nutrient deficiency and the existence of some types of alcohols produced by ethanol during fermentation, lead to changes in the yeast’s morphology – from single-cell it mutates into filamentous-cell yeast, that is, formed by a chain of cells, many of which are very elongated and ‘deformed’, – which can make up for the stress-provoked loss of productive efficiency. “This is how the yeast escapes from an unfavorable environment”, says Sandra.
The researcher has studied this mutation in the alcohol fermentation process, where the yeast undergoes tremendous stress when it is in the fermentation vats. Although this data is not conclusive yet, the researcher points out that filamentation indicates that yeasts have an adaptation advantage, because their surface area increases. “The yeast mutates from a single cell into a long filament”, says Sandra, who developed this research study with funding from FAPESP under the regular funding program. She began working on the project in 2005 and concluded it in 2008. As the cellular area increases, higher contact occurs because of the culture. This leads to compensation due to stress-caused cell death. “In a situation in which the yeast is stressed and does not mutate into a filament, the harm caused by stress can be more serious”, says the researcher. She points out that even under stressful conditions, some Saccharomyce strains do not change their morphology. The explanation is that this change could be a genetic characteristic, but it is still unknown whether there is a specific gene related to filamentation. “Various genes could be involved in this process”.
In the researcher’s opinion, the fact that this characteristic is found in industrial yeasts means that it has to have some kind of function, because it appears through selective pressure. “At first, I thought that this was a negative characteristic, but then I realized it was important”.
The Projects
1. Estresse, transporte e metabolismo de alfa-glicosídios em Saccharomyces cerevisiae (nº 04/10067-6); Modality Theme Project; Coordinator Pedro Soares de Araújo – USP; Investment R$ 482.204,54 (FAPESP)
2. Otimização da fermentação de sacarose e produção de álcool por Saccharomyces cerevisiae; Modality Technological Development and Innovation; Coordinator Boris Ugarte Stambuk – UFSC; Investment
R$ 173.005,92 (CNPq)
3. Aspectos básicos e aplicados da utilização industrial de leveduras (nº 05/01498-6); Modality Regular Funding of Research Project; Coordinator Cecília Laluce – Unesp; Investment R$ 118.245,46 (FAPESP)
Scientific Articles
BATISTA, A. S. et al. Sucrose fermentation by Saccharomyces cerevisiae lacking hexose transport. Journal of Molecular Microbiology and Biotechnology. v. 8, p. 26-33. 2004.
BADOTTI, F. et al. Switching the mode of sucrose utilization by Saccharomyces cerevisiae. Microbial Cell Factories. v. 7. 2008.