 hercules and antaeus, luca signorelli, 1490The end of last year witnessed the appearance of the first strong indications that men could also benefit from anti-human papilloma virus/HPV vaccine. This vaccine is currently approved in many countries, including Brazil, for young women as one of the therapies for the prevention of cervical cancer, the second most lethal tumor in women world-wide. Preliminary results of the clinical trials on the Gardasil vaccine, conducted since 2005 on three thousand heterosexual males suggest that the product also offers sound protection for the male gender. Gardasil is the trade name of the prophylactic vaccine developed by Merck Sharp & Dohme Laboratories for four types of HPV. “In 90% of the cases, the vaccine prevented the onset of genital warts and in 86% it avoided the onset of infections”, says Luisa Villa, director of the Brazilian branch of the Ludwig Cancer Research Institute. Villa has already coordinated trials with the prophylactic on women and acted as scientific consultant during the tests conducted on men in Brazil. “The data is still not conclusive and we have to wait another one to two years until we have a more detailed analysis”. Funded by Merck, the clinical trials, conducted on individuals without any previous history of HPV, ranging from the ages of 16 and 26, are also being conducted in several countries around the world, including Brazil. Homosexuals are also taking part in the trials, but no specific information on the action of the vaccine on this population has been disclosed yet.
hercules and antaeus, luca signorelli, 1490The end of last year witnessed the appearance of the first strong indications that men could also benefit from anti-human papilloma virus/HPV vaccine. This vaccine is currently approved in many countries, including Brazil, for young women as one of the therapies for the prevention of cervical cancer, the second most lethal tumor in women world-wide. Preliminary results of the clinical trials on the Gardasil vaccine, conducted since 2005 on three thousand heterosexual males suggest that the product also offers sound protection for the male gender. Gardasil is the trade name of the prophylactic vaccine developed by Merck Sharp & Dohme Laboratories for four types of HPV. “In 90% of the cases, the vaccine prevented the onset of genital warts and in 86% it avoided the onset of infections”, says Luisa Villa, director of the Brazilian branch of the Ludwig Cancer Research Institute. Villa has already coordinated trials with the prophylactic on women and acted as scientific consultant during the tests conducted on men in Brazil. “The data is still not conclusive and we have to wait another one to two years until we have a more detailed analysis”. Funded by Merck, the clinical trials, conducted on individuals without any previous history of HPV, ranging from the ages of 16 and 26, are also being conducted in several countries around the world, including Brazil. Homosexuals are also taking part in the trials, but no specific information on the action of the vaccine on this population has been disclosed yet.
Up to now, the expensive anti-HPB vaccines, given in three shots over six months at a total cost, in Brazil, of more than R$ 1 thousand, were part of the female health care segment. It was a somewhat marginal issue among men, and rightly so. As has been widely reported in medical literature, the infections and lesions caused by the human papilloma virus of the 16 and 18 types, two of the four HPVs in Merck’s prophylactic vaccine, are linked to 70% of the cervical cancer cases (the vaccine also protects women from the HPV-6 and HPV-11 types, linked to genital warts). This type of tumor is diagnosed in more than half a million women world-wide every year; 80% of the cases are diagnosed in poor or underdeveloped countries. It is estimated that 290 thousand women die every year from cervical cancer. In the male population, HPV infections have not been thoroughly researched yet, but they can lead to rare types of cancer, such as penile cancer (2% of the neoplasm cases among men in Brazil) and anal cancer (more common among homosexuals.) In view of initial studies showing the link between HPV and tumors in the genital organs in men and the preliminary results of the quadrivalent vaccine in clinical trials with individual males, Merck has recently put in a request for approval by the Food and Drug Administration (FDA), the US government agency that oversees the sales of food products and medical drugs, to give Gardasil to boys and young males from the ages of 9 to 26 years in order to prevent warts and other genital lesions. The FDA’s approval is still pending.
Although discussion in the medical community on the efficacy and high cost of immunizing millions of women with anti-HPV vaccines is far from reaching a consensus, men began to be the latest focus of research studies on the papilloma virus. A study published in August last year in the Cancer Epidemiology Biomarkers & Prevention journal showed that 65% of the men had some form of HPV. Nearly one half of the infected participants carried types of cancer-causing viruses combined with non-cancerous forms of the HPV. The HPV-16, HPV-51 and HPV-59 were the most common types of cancerous papilloma viruses found in the men participating in the study. The study involved the search for the existence of the virus in the genital organs of 1,200 men from the United States, Mexico and Brazil, ranging from the ages of 18 to 70 years, without a past history of sexually transmitted diseases or HPV infections. The overall prevalence of the virus was higher in Brazil (72.3%) than in the United States (61.3%) and Mexico (61.9%).
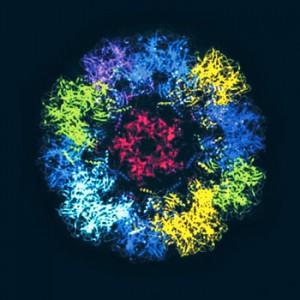
CDCParticles similar to the HPV in the vaccine: prevention of cervical cancerCDC
Risk factors
Sexual promiscuousness, lack of personal care habits, and lack of access to the public health network are factors that increase the risk of the male population (and of the female as well) of contracting HPV. “The fact that there are more circumcised men in the United States might be a protection factor for that population against the virus”, says Luisa Villa, one of the authors of the study prepared in conjunction with Mexican and American colleagues. Circumcision, a common practice among religious Jews, involves the removal of the foreskin (prepuce), the flap of skin that covers the gland of the penis. This removal facilitates cleaning of the penis and seems to reduce the HPV infection levels. The use of condoms during intercourse, a highly recommended procedure, reduces the chance of a person being infected and spreading the virus, but does not eliminate the risk entirely. The HPV is transmitted by means of direct contact with the infected skin and the virus hides in places not covered by the condom, such as the scrotum. There are approximately 200 types of HPV, 15 of which are associated with the onset of tumors. Nobody questions the fact that the association between HPV and cervical cancer is very strong and holds true in nearly 100% of the cases. In men, the link between the virus and penile and anal tumors is also strong.
A research study conducted by Rio de Janeiro’s National Cancer Institute/Inca and Fiocruz, published last year, helped define the role of HPV in typically male tumors. The Rio researchers showed that the papilloma virus was found in 75% of the 80 cases of penile cancer analyzed by them. “Previous studies had shown the link between the virus and this form of cancer in only 40% of the cases”, says Antônio Augusto Ornellas, an urologist at Inca and one of the authors of the paper. “Nowadays we have more refined techniques, such as the PCR, which make it easier to find the DNA of the virus in the cancerous tissues”. According to Ornellas, there are very few studies on HPV and penile cancer because this kind of tumor is very rare and is more common in underdeveloped countries. “It took us four years to find these 80 cases”, says the urologist. Based on this data, the researcher says he is in favor of immunizing men and women with anti-HPV vaccines. In addition to Merck’s Gardasil, another vaccine, Cervarix, manufactured by GlaxoSmithKline, is available in several countries, including Brazil. Cervarix provides immunity against the HPV-16 and HPV-18 types, the viruses most closely associated with cervical cancer. “The problem is the price of these vaccines”, says the doctor from Inca.
The price is the most obvious problem, but it is not the only one. Critics of the mass prescription of this expensive anti-HPV vaccine voice a number of arguments to defend their position, which is more cautious then the attitude of other physicians and researchers. The vaccines, whether the one manufactured by Merck or the one manufactured by GlaxoSmithKline, are theoretically able to prevent at most 70% of the cases of cervical cancer, and do not eliminate the need for women to undergo the Papanicolau test, the traditional and efficient preventive exam to detect this form of cancer. In addition, nobody knows how long the immunity provided by the vaccine will last, as it has been tested for only ten years. It is still not entirely clear if the vaccine is really efficient for the older female population, already exposed to the papilloma virus, although there is some data available in this respect, stemming from results of trials on women from the ages of 26 to 45 years.
Cost/benefit ratio
There is a general consensus on one point: young people who have not yet become sexually active should be the priority population for this kind of vaccine. A study published in the August 21 issue of the New England Journal of Medicine, a respected medical journal from the United States, questions the generalized immunization of the female population with the existing anti-HPV vaccines. “The cost/benefit ratio of HPV vaccination will depend on the time of duration of the protection offered by the vaccines and will be better optimized through the immunization of pre-adolescent girls; efforts in this respect should be focused on females up to the age of 18 or 21 years”, stated Harvard University’s Jane J. Kim and Sue J. Goldie, who wrote the article. If an extra reinforcing dose becomes necessary in the course of womens’ lives, immunization might be too expensive to be borne by the public health system, and it might be advisable to invest more in the Papanicolau test. “In spite of the great expectations and the promising results of the clinical trials, we still do not have enough evidence to state that we have an efficient vaccine against cervical cancer”, said researcher Charlotte J. Haug, the editor of the Journal of the Norwegian Medical Association, in an editorial published in the afore-mentioned issue of the New England Journal of Medicine.
 leonardo da vinci/reproductionArticles in the press, such as the one signed by Elisabeth Rosenthal in last year’s August 21st issue of the New York Times, describes the advertising and pressure tactics put on by the pharmaceutical industry on physicians, politicians, means of communication and the population in general to promote the anti-HPV vaccine. In addition to referring to doubts about the real efficiency of the vaccine and the possible side effects, the reporter mentions physicians and nurses who were allegedly paid US$ 4,500 by Merck to give promotional lectures on the vaccine and cervical cancer. She also mentions researchers who have conducted scientific research studies on Gardasil and are paid fees or consultancy fees by the manufacturer. Fortunately, the top scientific journals nowadays ask the authors of accepted papers to specify cases of conflict of interest. The article’s conclusion states that now the pharmaceutical labs want to expand the target audience of the vaccine: they want to get the approval for its use on older women and young males.
leonardo da vinci/reproductionArticles in the press, such as the one signed by Elisabeth Rosenthal in last year’s August 21st issue of the New York Times, describes the advertising and pressure tactics put on by the pharmaceutical industry on physicians, politicians, means of communication and the population in general to promote the anti-HPV vaccine. In addition to referring to doubts about the real efficiency of the vaccine and the possible side effects, the reporter mentions physicians and nurses who were allegedly paid US$ 4,500 by Merck to give promotional lectures on the vaccine and cervical cancer. She also mentions researchers who have conducted scientific research studies on Gardasil and are paid fees or consultancy fees by the manufacturer. Fortunately, the top scientific journals nowadays ask the authors of accepted papers to specify cases of conflict of interest. The article’s conclusion states that now the pharmaceutical labs want to expand the target audience of the vaccine: they want to get the approval for its use on older women and young males.
Nevertheless, and in spite of such doubts and the vaccine’s limitations, many serious researchers in Brazil and abroad are in favor of immunizing more women, and perhaps even young boys, before they become sexually active. In Brazil, the public health system has not yet included the vaccine in its schedule, but the product is available at private clinics for those who can afford the price. “Vaccinating men is also a way of protecting women from contagion by the virus”, says Edison Fedrizzi, a professor of gynecology and obstetrics at the Federal University of Santa Catarina/UFSC and head of one of the centers in Brazil that is testing the quadrivalent vaccine on both males and females. “In this way, we will achieve the so-called herd immunity”. He further argues that some of the doubts related to the anti-HVP vaccine also surrounded other vaccines, such as the one for B-type hepatitis, and were mitigated in the course of time. “Existing vaccines are not perfect, but we cannot afford to simply wait for the ideal vaccine”, says Luisa Villa, who is going to head the recently created National Institute of Science and Technology/INCT of Papilloma virus-caused Diseases, which has received funding in the amount of R$ 7 million (half of which came from FAPESP and half from the federal government). “Women are dying from cervical cancer”. In 2008, approximately 19 thousand new cases appeared in Brazil, according to
Inca.
There are some initiatives in course in this battle against the link between the papilloma virus and tumors. Vaccines able to immunize people against up to nine new types of HPV are currently being tested. In São Paulo, the Instituto Butantan institute, one of the major vaccine production centers in Brazil, has been working on a national version of the anti-HPV vaccine for four years. “The anti-papilloma virus vaccine is similar to the Type B Hepatitis vaccine”, says Paulo Lee Ho, director of the Butantan’s biotechnology center. “We are already able to produce it in the lab, but the problem is to produce the vaccine on a commercial scale”. Before the Brazilian-made vaccine is ready, a new international vaccine is scheduled to be tested in Brazil in 2010. The new formula has an ambitious objective: not only to prevent, but also to cure cervical cancer.
The search for the injection that cures
Vaccine designed to prevent cervical cancer is expected to be tested in São Paulo next year
In 2010, Brazil is expected to be the first country in the world to test a candidate for the anti-HPV vaccine with very unique characteristics: the vaccine will not only prevent infections caused by the virus, thus preventing the onset of cervical cancer in non-infected women, but will also eradicate the pathogen and the tumors in patients with signs of cervical cancer. Developed by researcher Robert Garcea and his team from the University of Colorado, and funded by the Bill & Melinda Gates Foundation, the new vaccine’s ambition is to be a preventive and curative tool at the same time; it is also expected to be very low cost (a few dollars per dose), unlike the expensive immunization vaccines launched by commercial labs. The target audience for this new vaccine is comprised of developing countries, where personal care habits and precarious medical services make HPV a huge risk factor for the incidence of cancer, and which do not have enough money to finance the existing vaccines. “This vaccine is currently being made by the BioSidus company in Buenos Aires and will probably be ready for clinical trials in São Paulo in 2010”, says Garcea, in an interview by e-mail to Pesquisa FAPESP. Initial tests, under the responsibility of the team headed by Luisa Villa, of the Ludwig Institute, will verify if the vaccine is safe for women with persistent infections, caused by the HPV-16, in low grade lesions of the cervix. This kind of papilloma virus is responsible for a number of preliminary lesions and for 50% of the cases of cervical cancer.
The vaccine could be a development of the vaccines that are already on the market. To induce the immune system´s response to the HPV, the existing vaccines inject a formula into the body that is very similar to the virus itself, and is referred to as virus-like particles or by the acronym VLP. The L1 protein is part of the VLP, and is the main component of the “cover” that covers the genome of the HPV. The formula, however, does not contain the pathogen’s DNA. In this way, the vaccine, which is comprised of a virus without its genetic material, is inoculated into the organism and provokes the same immune reaction which the contact with the HPVG itself would bring on. The organism produces specific antibodies against the HPV type used to manufacture the immunizing agent. In the commercial vaccines, the L protein is obtained from yeast or insect cells, with the help of genetic engineering. In the case of the new vaccine, scientists believe that they have developed a more efficient and less expensive way of getting good immunization. They resort to the well-known Escherichia coli bacteria as a means of synthesizing the L1 protein, which is combined with another protein, the E7. This method allegedly has two major advantages: it would result in a vaccine that would be easier to purify, and that could be stored in powder form, thus avoiding refrigeration expenses to preserve it; the combination of the two proteins in the vaccine would generate an immune response able even to eradicate existing infections.
The vaccine worked very well when it was tested on rodents. If it is efficient against the HPV-16, the vaccine – developed thanks to funds donated by the owner of Microsoft – might quickly incorporate more formulas to attack all of the cancer-causing papilloma viruses. This is what the researchers are hoping for.
Republish