Eduardo CesarEpidendrum denticulatum: ongoing diversificationEduardo Cesar
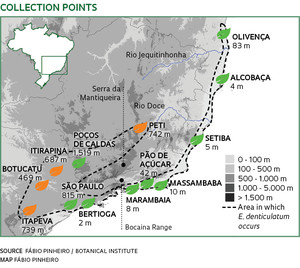
Pink orchids, still considered to be the same species, grow in dunes near Marambaia beach, Rio de Janeiro, and Alcobaça beach, in the state of Bahia. Although they are separated by 900 kilometers, they should produce seeds if their reproductive cells one day meet. However, no embryo formed after pollination between plants from the two sites, induced by botanists in São Paulo. Other representatives of the same species of orchid, Epidendrum denticulatum, from the Cerrado in the municipality of Itirapina in São Paulo State, and in the municipality of Peti, in Minas Gerais, have also turned their backs on one another. The four groups of orchids seem to be following different evolutionary paths and are perhaps already forming different species, even though they are identical in size, flowers, colors and external structures.
“We are seeing something very rare, the emergence of new species,” says Fábio Pinheiro, a researcher at the Botanical Institute of São Paulo. He detected the formation of species—or speciation—among representatives of the same species. “Darwin had already mentioned variations between species, but could not imagine that they were relevant enough to prevent reproduction between representatives of different populations of the same species.” In 2010, Pinheiro collected pollen and induced cross pollination between 258 specimens of 13 populations of Epidendrum denticulatum found in forests inland and on the coast of the states of Bahia, Espírito Santo, Minas Gerais, Rio de Janeiro and São Paulo, kept in the institute’s nursery. Some populations, even from the same ecosystem, had accumulated genetic differences that prevented the formation of viable embryos. The inability of reproductive cells of the same species to generate fertile offspring—reproductive incompatibility—”is one of the first steps of genetic differentiation that, in thousands of years, may lead to a new species,” he says.
He examined the separation between lineages of a single species, whereas the usual approach compares different species, after they have formed. “This approach not only allowed Pinheiro to quantify the degree of isolation between new lineages, but also to associate these early stages of differentiation with historical expansion and fragmentation of forests and fields, which catalyzed the differentiation between populations and shaped the observed patterns of reproductive isolation,” says Salvatore Cozzolino, a specialist in orchids from the Mediterranean region and professor at the Federico II University of Naples, Italy, where the Brazilian botanist performed some of his analyses. “Recognizing the early stages of reproductive isolation involved in the formation of new species is an important step to understanding how the extraordinary biodiversity of Brazil, and the tropics in general, is generated and maintained.”
 Photos Eduardo CesarIn parallel, a study of two species of bromeliads from Sugar Loaf Mountain and other rocky formations in the city of Rio de Janeiro—one with white flowers and the other with red flowers—further demonstrated the tortuous paths along which living beings evolve. According to a classical theory, in order to be considered the same species, organisms must exchange genes among themselves and not with representatives from other species. However, the analyses of Clarisse Palma Silva, of the Universidade Estadual Paulista (Unesp) in Rio Claro, indicated that populations of the same species of bromeliads are already quite different genetically and very rarely exchange genes with each other, even though they live in nearby hills. Moreover, the different species of bromeliads at one site exchange genes, forming fertile hybrids that can breed among themselves and with representatives of the pure species from which they originated. The unexpected governs evolution.
Photos Eduardo CesarIn parallel, a study of two species of bromeliads from Sugar Loaf Mountain and other rocky formations in the city of Rio de Janeiro—one with white flowers and the other with red flowers—further demonstrated the tortuous paths along which living beings evolve. According to a classical theory, in order to be considered the same species, organisms must exchange genes among themselves and not with representatives from other species. However, the analyses of Clarisse Palma Silva, of the Universidade Estadual Paulista (Unesp) in Rio Claro, indicated that populations of the same species of bromeliads are already quite different genetically and very rarely exchange genes with each other, even though they live in nearby hills. Moreover, the different species of bromeliads at one site exchange genes, forming fertile hybrids that can breed among themselves and with representatives of the pure species from which they originated. The unexpected governs evolution.
Recent studies strongly based on genetics indicate that the processes observed in orchids and bromeliads also occur in other plants and animals. The phenomena being described show the fragility of the supposed rules governing one of the basic biological processes, speciation. Now we see that this process occurs through more diverse mechanisms than previously believed. Both with plants and animals, individuals that should normally be able to reproduce lose their reproductive affinity, sometimes as a consequence of a tiny genetic modification, and those not expected to be able to reproduce generate offspring, which are often fertile. Subtle genetic differences can make reproduction between morphologically identical individuals impossible. Sometimes, however, even large genetic differences between lizards or amphibians do not prevent distantly related individuals from interbreeding and—sometimes rapidly —forming new species.

CÉLIO HADDAD/UNESPThe triploid Phyllomedusa híbrida (39 chromosomes): an unlikely organism, according to the classical rules of evolutionCÉLIO HADDAD/UNESP
Fragile definitions
The new findings complicate something that was already not easy to understand, starting with the concept of species, as basic to biology as the gene is to genetics. “We still do not have a good definition of species,” says zoologist Célio Haddad, professor at Unesp, Rio Claro. Since 1859, when Charles Darwin published his book The Origin of Species and acknowledged that the distinction between species and lineage—or populations—was vague and arbitrary, the situation has not improved much. In 2007, Kevin de Queiroz, a biologist at the Smithsonian Institute in Washington, reviewed various definitions of species—biological isolation or recognition, the ability to live in the same space, spatial or genetic cohesion or a common evolutionary history—and acknowledged that all of them were imperfect.
“The most conservative approach, which considers only morphological aspects, tends to gather several species into one,” says Haddad, “while integrative taxonomy, which is more modern, detailed and accurate, also considers variations in DNA and behavior, and tends to split species up.” He notes that the concept of species varies case by case, according to the criterion and viewpoint. The orchids that do not reproduce with each other can be seen in three ways: as a species differentiating, as representatives of different species, or as semi-species, a concept that the zoologist Ernst Mayr introduced in 1963 to define the populations of animals or plants that have not yet completed the differentiation process.
 Infographic: Ana Paula Campos; Map: Fábio PinheiroHaddad believes that the reproductive incompatibility seen in orchids and bromeliads is probably common in animals too. “We just haven’t evaluated them properly.” There are few examples. We have seen that the populations of a species of plant with white flowers in a region near the Arctic, Draba fladnizensis, had total reproductive incompatibility. Populations of marine crustaceans known as copepods—from the east and west coasts of the United States—can no longer produce fertile offspring. Other marine invertebrates, called bryozoans, have formed genetically incompatible populations along the coast of European countries.
Infographic: Ana Paula Campos; Map: Fábio PinheiroHaddad believes that the reproductive incompatibility seen in orchids and bromeliads is probably common in animals too. “We just haven’t evaluated them properly.” There are few examples. We have seen that the populations of a species of plant with white flowers in a region near the Arctic, Draba fladnizensis, had total reproductive incompatibility. Populations of marine crustaceans known as copepods—from the east and west coasts of the United States—can no longer produce fertile offspring. Other marine invertebrates, called bryozoans, have formed genetically incompatible populations along the coast of European countries.
Studies like these “open up a window to the different mechanisms involved in the formation of new lineages,” says Samantha Koehler, a researcher at the Federal University of São Paulo (Unifesp) who specializes in orchids. And they show how differences in DNA can result in the formation of new species and recalibrate the rules of evolution. For these orchids and bromeliads, reproductive incompatibility seems to be more decisive than a factor that Darwin saw as fundamental to speciation—geographic isolation, whereby geographically distant populations could differentiate to the point of forming new species.
“The basis of speciation is isolation, but the isolation mechanism is not necessarily geographical,” says Mário de Pinna, a researcher at the Zoology Museum of the University of São Paulo (USP). “Reproductive isolation is the result of some factor that prevents the flow of genes, which may be geographical isolation or some biological or local contingency resulting in the segregation of part of a population.”
Distance no longer seems to be as important in preventing reproductive affinity between Epidendrum populations. Populations separated by a distance of a thousand kilometers were still able to interbreed and form viable embryos, while others in the same location were no longer able to do so.
“Reproductive isolation is what will effectively separate the lineages,” reiterates Rodrigo Marques Lima dos Santos, a researcher at the USP Biosciences Institute and professor at the University of Mogi das Cruzes (UMC), who studies speciation in lizards. Distantly related species can interbreed—even individuals from different taxonomic genera—resulting in fertile hybrids. Previously considered unlikely, these hybrids indicate that geographical isolation and the accumulation of genetic differences over thousands of years are not sufficient for reproductive isolation. Again expanding the classical view of evolution, the emergence of new species can also be immediate, even without intermediate phases. “Two distinct—but chromosomally compatible—species of lizards could crossbreed, yielding hybrid young that could already be a new species,” he says. “If the hybrid is feasible, it will be viable and sometimes better than its parents.”
Leading to their own death
Similar to mongrel dogs, which tend to be stronger and more resistant to diseases than pure-bred dogs, lizard hybrids are usually more active than their parents, more competitive for food and space, and can force parents to extinction because they all live in the same space. “The parent species are creating a strong competitor, which can lead to their death,” says Santos. Sometimes, when determining the lines of kinship between animals, he found only hybrids, rather than the species that most probably led to them. “Despite the problems involved in sampling and human impact driving species to extinction, hybridization can also reduce biodiversity, as species merge and then disappear.”
Frogs can also result from unlikely matings. This is the case of a green frog from the forests of southeastern and southern Brazil that was named Phyllomedusa tetraploidea because of a rare feature among vertebrates: each cell of this species harbors four copies of each chromosome, i.e. is tetraploid (humans and most vertebrates are diploid, meaning they have two copies).

CLARISSE PALMA-SILVA/UNESPBromeliads in Rio de Janeiro: the Pitcairnia albiflos… CLARISSE PALMA-SILVA/UNESP
Haddad and other biologists concluded that the new species must be the result of crossbreeding between males and females from a diploid species, Phyllomedusa distincta, or from a common ancestor. It is tetraploid because the originating gametes (sperm and ova) were diploid; normally they are haploid, with only one version of each chromosome. The gametes will produce descendants with 52 chromosomes in each cell, double the 26 chromosomes of the parent species. “This is a highly unlikely phenomenon,” says Haddad, “but in millions of years of evolution there is some chance of it happening.”
P. tetraploidea can mate with the parent species and form triploid hybrid frogs with 39 chromosomes in each cell. The descendants (triploid) try to mate with the parent species (diploid), but are sterile because their gametes are defective. Sometimes, however, the result can be an animal that Haddad calls almost sterile: the chromosomes are organized enough to enable the production of a few viable gametes, “ignoring the classic definitions of reproductive isolation between different species,” he says.
But why are evolutionarily distant individuals able to mate with each other and very similar individuals no longer can? What differences are relevant? Santos believes that reproductive compatibility can be determined by the number and shape of chromosomes, since lizard species with a genetic similarity of only 85% can produce fertile offspring if they are chromosomally compatible. “Humans and chimpanzees are more than 98% similar, genetically, but do not reproduce with each other largely as a result of deletions, fusions and chromosomal rearrangements,” he says. “Humans have 46 chromosomes and chimpanzees have 48, and about 10 large chromosomal inversions also separate the species. It is sufficient for reproductive isolation.”
Chromosomes, songs and smells
Among animals, subtle changes in behavior—in the song of birds or croaking of amphibians—can hinder recognition and mating between species, inducing differentiation of lineages. Reproductive incompatibility can also result from morphological differences—expressed, for example, in the size of sexual organs. This prevents a Saint Bernard dog from mating with a poodle, although they may cross with breeds of intermediate size, since all 400 breeds of dogs have the same number of chromosomes.

CLARISSE PALMA-SILVA/UNESP… and P. staminea, which form hybrids, and Sugar Loaf Mountain, one of the hills on which they live CLARISSE PALMA-SILVA/UNESP
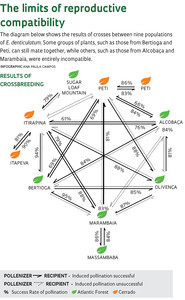
Among plants, changes in odor may fail to attract pollinating insects and serve as a barrier to reproduction. In the study with orchids, genetic differences were once again most influential, in a surprising way. Most crossbreeding between Epidendrum plants showed an asymmetrical pattern: pollen taken from a flower in one location did not fertilize a flower from another location, but fertilization in the reverse direction worked (see diagram). “The asymmetrical reproduction pattern is typical of the early stages of speciation, when distinct lineages begin to differentiate,” says Pinheiro. “The asymmetrical reproductive pattern must be common in nature,” says Fábio de Barros, coordinator of the orchid nursery.
This phenomenon may be the result of incompatibility between the DNA in the nucleus of reproductive cells and DNA from the non-nuclear chloroplast, the egg from the recipient plant. “One almost always thinks just of the DNA in the nucleus, but it is the variation in chloroplast DNA that determines embryo viability and compatibility between populations,” he concluded, after analyzing the seeds of all crosses using nine markers for nuclear DNA and six for chloroplast DNA.
Small sections of the genome or even a few genes may determine if a species will form or be preserved. “The organisms must be able to easily exchange sections of the genome that facilitate their adaptation, but genes or islands of speciation, which define the characteristics of a species, such as the color of the flowers, are not exchanged,” says Silva. This viewpoint would explain why species of bromeliads in the hills of Rio de Janeiro form distinct white flower and red flower varieties, even when forming hybrids.
“We must take advantage of the privilege of being in an extremely diverse country and collect more data on reproduction and pollinators, thus defining the environmental factors that might contribute to differentiation in lineages,” suggests Koehler, from Unifesp. That is what Pinheiro intends to do, as he is planning to transport Epidendrum lineages from the coast to the Cerrado, inland, to test his hypothesis that geographical isolation is a consequence, not a cause of speciation. If it works, he will help update Darwin’s ideas in a different way.
Projects
1. Phylogeography of the species of Epidendrum (Orchidaceae) belonging to the Atlantic clade (subgenus Amphiglottium) (09/15052-0); Grant Mechanism Post-doctorate. Coordinator Fábio Pinheiro – Botanical Institute; Investment R$280,131.37 (FAPESP).
2. Speciation, reproductive isolation, and population genetics in species of the Bromeliaceae family: implications for taxonomy, evolution and conservation (09/52725-3); Grant Mechanism Biota Program – Young Investigators Awards; Coordinator Clarisse Palma da Silva; Investment R$441,491.60 (FAPESP).
3. Speciation of frogs in high-altitude environments (08/50928-1); Grant Mechanism Thematic Project; Coordinator Célio Haddad – Unesp; Investment R$1,407,985.13 (FAPESP).
Scientific articles
GRUBER, S. L. et al. Cytogenetic analysis of Phyllomedusa distinct Lutz, 1950 (2n = 2x = 26), P. tetraploidea Pombal and Haddad, 1992 (2n = 4x = 52), and their natural triploid hybrids (2n = 3x = 39) (Anura, Hylidae, Phyllomedusinae). BMC Genetics. v. 14, n. 1, p. 75, 2013 (online).
PINHEIRO, F. et al. Phylogeographic structure and outbreeding depression reveal early stages of reproductive isolation in the Neotropical orchid Epidendrum denticulatum. Evolution. v. 67, p. 2.024-39, 2013.
PALMA-SILVA C. et al. Sympatric bromeliad species (Pitcairnia spp.) facilitate tests of mechanisms involved in species cohesion and reproductive isolation in Neotropical inselbergs. Molecular Ecology. v. 20, 3.185-201, 2011.