Brazil, the world’s largest beef exporter, is taking its first steps into a market that has attracted tens of millions of dollars in recent years: alternative meat products that taste, smell, and feel like the real thing. There are two paths to this goal: growing meat in a laboratory from animal stem cells, also known as clean or cultured meat, or creating a vegetable-protein product that emulates red meat. In May of this year, Rio de Janeiro–based foodtech Fazenda Futuro launched the Futuro Burger, a vegetable burger that claims to look and taste like real beef, and costs roughly R$17 for a pack of two burgers. The company is owned by entrepreneur Marcos Leta, who also founded successful juice company Do Bem.
Leta is following the path already taken by many foreign companies that offer plant-based alternatives to animal products. The main ingredients of the Futuro Burger are isolated soy, chickpea, and pea protein. The reddish color comes from beet juice. According to Leta, the burger is not only similar to beef in appearance, but also in taste, texture, and smell. An artificial tongue, an electronic device with taste sensors that mimic the functioning of the human organ, was used in the development process. “The nutritional value is also very similar,” says Leta. This was achieved by analyzing the best combinations of plant proteins and lipids, with the help of artificial intelligence.
The Futuro Burger is Brazil’s first vegetable burger designed to emulate red meat
Ingredients
The product is based on isolated soy, chickpea, and pea protein
Appearance
Its reddish, blood-like color comes from beet juice
Nutritional value
The company used artificial intelligence to research the best combination of proteins and lipids
Growing Market
In the USA, sales of plant-based meat alternatives hit US$670 million in the first half of 2018
Clean meat
As well as plant-based burgers, another new alternative being explored is to grow meat from stem cells in a lab
The goal is to reach not only vegetarians, but also people seeking a more ecologically sustainable diet. A study released by the Climate Observatory in 2016 showed that the agricultural sector accounts for 69% of greenhouse gas emissions in Brazil. “We want to show that it’s possible to revolutionize the food industry without negatively impacting the environment,” says Leta.
During development of the burger, the company consulted with The Good Food Institute (GFI), a US-based nonprofit that specializes in alternative protein sources. “Our aim is to create a healthier, fairer, and more sustainable food system. Our team of scientists, entrepreneurs, and public policy experts is working to increase the food industry’s use of products made from plants or cultured animal cells,” explains Felipe Krelling, innovation and research coordinator for GFI in Brazil.
Although it is new in Brazil, beef-imitating vegetable burgers have been available in US stores for three years. Beyond Burger was the first company to sell plant-based burgers in supermarket chains across the country. This year, it became the first plant-based meat company on the American stock exchange Nasdaq. As well as burgers, it sells ground beef and sausages made from a combination of vegetable protein sources.
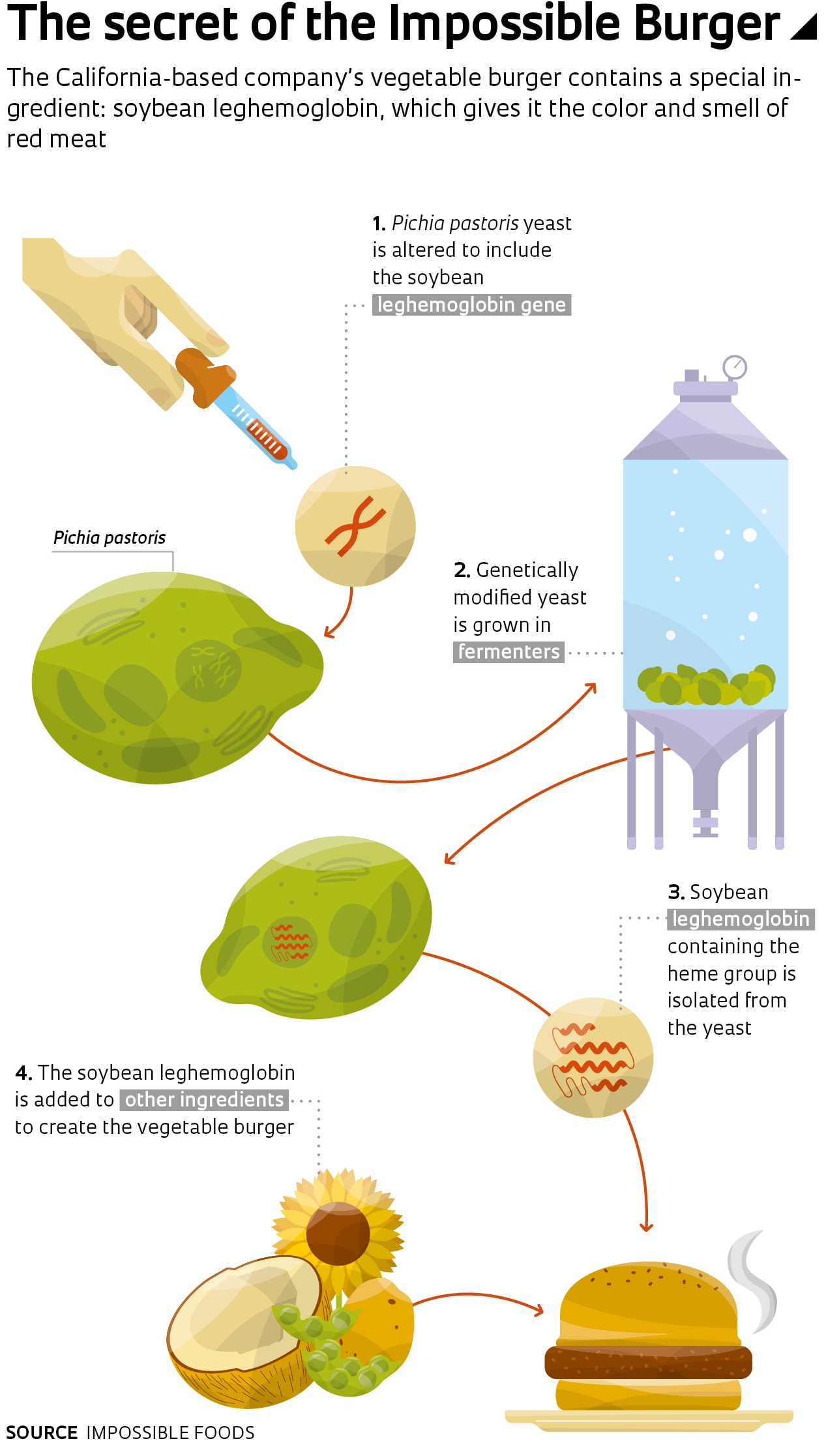
California startup Impossible Foods, meanwhile, has gone a step further. The red color of its burger comes from a genetically engineered protein similar to hemoglobin. The company’s researchers used a component of hemoglobin known as the heme group to recreate the red color of raw meat and the characteristic smell it generates when cooked. The heme group also occurs in a substance called leghemoglobin, found in legume roots. It has a very similar protein structure and function to hemoglobin.
Based on this knowledge, scientists at Impossible Foods devised a method to extract the heme group from soybean roots on an industrial scale. They genetically modified a yeast called Pichia pastoris so that it produces soybean leghemoglobin, allowing them to mass produce it by growing large quantities of the yeast in vats.

Léo Ramos Chaves
Research and development at Israeli startup Aleph FarmsLéo Ramos ChavesCultured meat
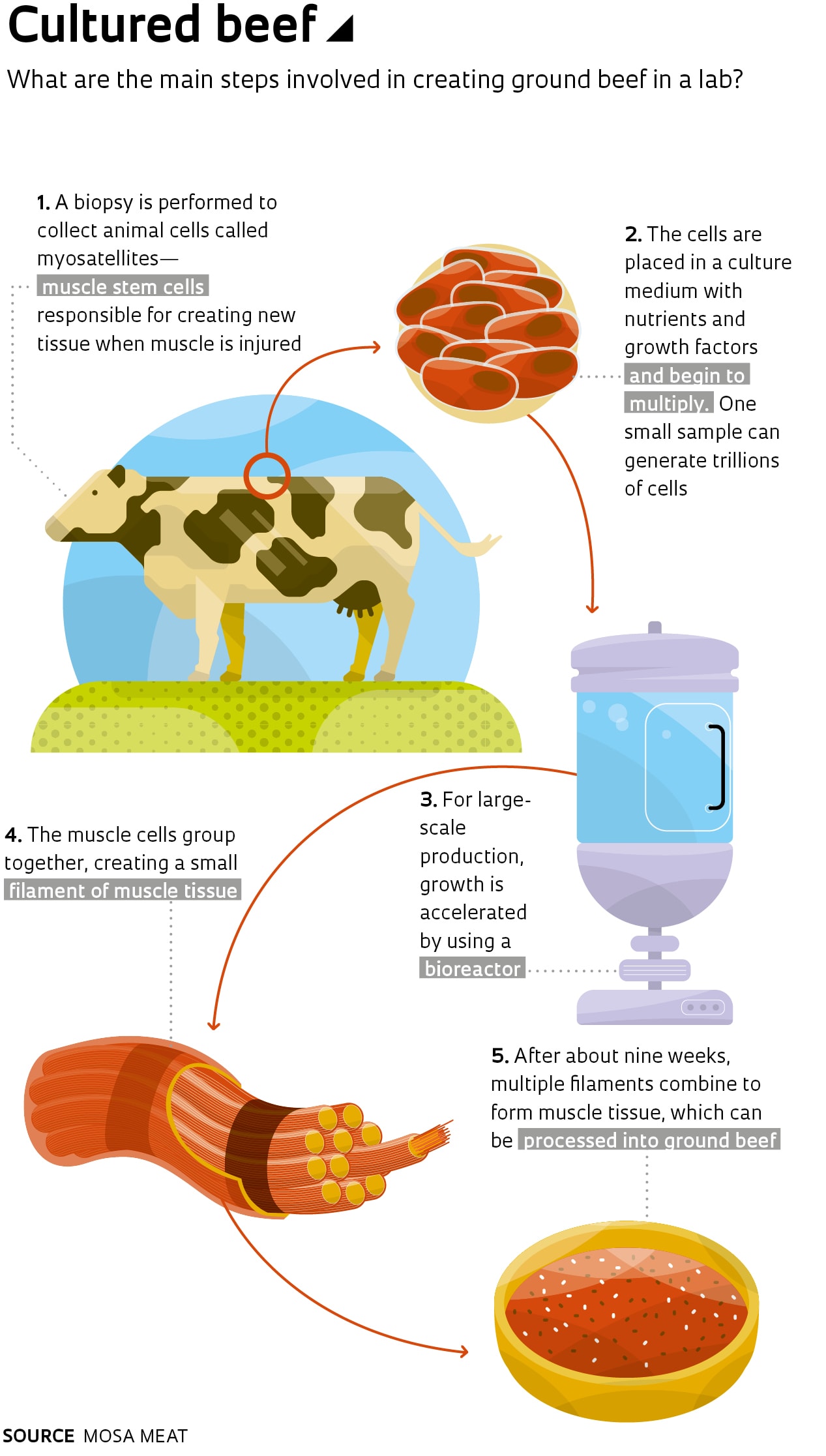
Entrepreneurs investing in synthetic meat grown from bovine stem cells, sometimes referred to as cell-based meat, have an even stronger argument for steak lovers: what they make is not just similar to meat—it is meat. The first lab-grown burger was created at Maastricht University in the Netherlands, based on research by physiologist Mark Post.
The studies began in 2008 and the result was presented at a press conference in London five years later. Three people who tasted the burger at the time—Post himself and two food experts—agreed that the meat was a little dry and lacking in flavor due to its low fat level, according to The New York Times.
The process developed by Post starts with the extraction of bovine stem cells from the muscle tissue of a cow. These undifferentiated cells are then multiplied in a culture medium containing nutrients and growth factors, transforming them into muscle fibers. Approximately 20,000 thin strips of muscle tissue are combined to form a burger weighing about 140 grams. Mass production would require the use of a bioreactor.
The Dutch burger cost €250,000 to make, funded primarily by Sergey Brin, one of the cofounders of Google. As part of his effort to get the product on the market, Post created Mosa Meat, a spin-off company from the University of Maastricht. In July 2018, the startup raised €7.5 million from the Bell Food Group—the biggest company on the Swiss meat market—and Dutch investment firm M Ventures to continue product development and begin marketing activities.

Mosa Meat/ Divulgação
Lab-grown burger from Maastricht University in the NetherlandsMosa Meat/ DivulgaçãoLate last year, Israeli startup Aleph Farms announced that it had created the first lab-grown steak. Didier Toubia, the company’s CEO, told Pesquisa FAPESP that he decided to begin researching cultured meat in 2016. The project came to fruition at the School of Biomedical Engineering of the Israel Institute of Technology (Technion).
The prototype, a small strip of steak weighing just a few dozen grams, cost US$50 to make, which is still high compared to traditional meat sold by butchers, but represents an improvement on the cost of the burger made at Maastricht. The product could hit supermarket shelves within five years.
The process of growing a steak in vitro is more complex and requires the cells to be organized three-dimensionally to provide volume and thickness. To achieve this, a support structure called a scaffold is needed, which is usually made of collagen—of animal origin. The problem is that the clean meat production process should not use any animal products. This has led to the emergence of several new technologies. Brazilian startup Biomimetic Solutions, founded at the Minas Gerais Federal Center for Technological Education (CEFET-MG), has created a plant-based scaffold.
The first cell-based burger was made in the Netherlands at a cost of €250,000.
“We created a plant-based synthetic polymer. We are one of the first companies in the world specializing in scaffolds for clean meat production,” says Lorena Viana, a master’s student at UFMG and one of the startup’s founders, along with materials engineers Ana Elisa Antunes and Alana Benzo, and CEFET-MG researchers Aline Bruna da Silva and Roberta Viana. “We decided to focus our business on the cultured meat market, where we have few direct competitors,” says Viana, the company’s commercial director. Mosa Meat and Aleph Farms are also developing their own scaffolds.
The Cellular Agriculture Society (CAS) was created in the US in 2016 to stimulate the field of cellular agriculture, which includes the development of cell-based meat. “Latin America is the only place in the world that has not yet developed lab-grown meat,” says Matheus Saueressig, a computer science student at the Federal University of Rio Grande do Sul (UFRGS) and director of communications for CAS in South America. “Brazil is the largest meat producer in the world and risks missing out on this new market,” he warns.
Startups devoted to cultured meat research and manufacturing, according to Saueressig, have been receiving heavy investment from venture capital firms and billionaires such as Richard Branson and Bill Gates, who both have stakes in California-based Memphis Meat, as well as multinationals in the food sector, such as Cargill, the Bell Food Group, and Tyson Foods. Major beef producers are supporting clean meat manufacturers to gain a foothold in the new market and avoid being left out if it is successful.
For now, plant-based products dominate the growing market for beef alternatives. A study by consultancy Nielsen found that Americans spent US$670 million on plant-based products designed to imitate meat in the first half of 2018—while vegans and vegetarians make up just 5% of the country’s population. Businesses attempting to produce meat from stem cells are targeting a much larger market. Beef is expected to generate global revenues of US$2.1 trillion by 2020, according to US consultancy Grand View Research.
Challenges ahead
Despite the market’s prospects, not all reactions have been positive. The term “clean meat,” for example, has been disputed by both vegans and farmers. The former are against using the adjective “clean” for a product that uses cells extracted from animals, while the beef industry opposes the use of the word “meat” for fear of competition. The American Farmers Association has asked the Department of Agriculture to prohibit products not made from traditionally raised and slaughtered animals from being called beef or meat—they want to differentiate the food they produce from the new alternatives entering the market. One of the biggest challenges faced by these new companies is not commercial but scientific: they need to ensure that the production process does not use any components of animal origin.
For the first ever lab-grown burger, Mark Post used fetal bovine serum to nourish the stem cells. Today, both Mosa Meat and Aleph Farms guarantee that they no longer use any ingredients derived from animals. However, veterinarian Flávio Vieira Meirelles, from the School of Zootechnics and Food Engineering at the University of São Paulo (FZEA-USP), believes it is very difficult to replace the amino acids, proteins, sugars, vitamins and growth factors found in animal blood with substances isolated from plants. “There are alternatives to fetal serum, but they also come from animals. And there are several other substances of animal origin involved in the different stages of the process,” he says. “It will be some time before cells can be cultivated on an industrial scale and at a viable cost without using any animal products whatsoever.”
Some foodtechs, such as American startups Just and Memphis Meats, are investing in lab-grown meat that does not even require a simple animal biopsy. They use stem cells harvested from feathers to produce chicken meat. Just sells plant-based alternatives to foods made from animal products, such as mayonnaise, while Memphis Meats is researching a range of different meats in the laboratory, including chicken and duck.
Same texture
Liz Specht, GFI USA’s associate director of science and technology, tasted Memphis Meat’s cultured duck meat in 2017. “What struck me most was the texture. When you bite into muscle fibers, they have a certain elasticity, and the cell-based meat I ate had the same quality,” she told the GFI shortly after the experiment. “The meat was breaded and served with a sauce, so it was a little difficult to judge the taste of the meat itself, but the texture was unmistakable.”
Another challenge for cell-based meat manufacturers is to prove that the product is safe for human consumption. “They will have to clearly identify what substances are used in the cell differentiation process, proving that the product is safe and nutritional,” says biochemist Viviane Abreu Nunes Cerqueira Dantas, from USP’s School of Arts, Sciences, and Humanities.

Impossible Foods / Divulgação
Impossible Foods factory: investment in researchImpossible Foods / DivulgaçãoThe researcher is suspicious of one claim commonly made by startups in the sector: that unlike traditional slaughtered meat, lab-grown meat does not require the use of antibiotics. “The first stages of large-scale cell-based meat production cannot be done without the use of antibiotics. I don’t know of any other substance that provides the same effect in the context of cultured meat production,” she says. According to Dantas, meat products, particularly those that undergo greater manipulation, offer an excellent culture medium for microorganisms due to their high humidity, near-neutral pH, and nutrient-rich composition.
GFI’s Felipe Krelling says that antibiotics can be used for a short time to minimize the risk of contamination when separating a cell line from a biopsy, in case it is contaminated with bacteria. “But there is no need to use antibiotics in any other part of the production process. The technology already exists in the bioprocessing industry. It can be adopted by cell-based meat companies to create an antibiotic-free environment for cell proliferation,” says Krelling.
Scientific article
LYNCH, J. and PIERREHUMBERT, R. Climate impacts of cultured meat and beef cattle. Frontiers in Sustainable Food Systems. Feb. 19, 2019.
