Applying a single dose of natural compounds to sugarcane crops can lead to subtle cell-wall changes that can significantly improve yields in second-generation ethanol, or ethanol produced from sugarcane bagasse and straw. A paper published last December in the journal Biomass and Bioenergy suggests that this can improve saccharification by 120% in 12-month-old sugarcane bagasse. Saccharification, also known as hydrolysis, is a chemical process that utilizes enzymes to convert complex carbohydrates such as cellulose and hemicellulose — which do not naturally ferment — into simpler sugars such as sucrose, which can be fermented to make ethanol.
“We conducted field trials using very low doses of these compounds, which were rapidly eliminated and still resulted in the desired changes,” says Wanderley Dantas dos Santos, a biochemist at the State University of Maringá (UEM) in Paraná and the lead author of the paper. “We observed no undesirable side effects; the plants developed normally without any effect on yields.” Santos has been investigating this approach, known as “physiological engineering,” since 2008 as part of his postdoctoral research under Marcos Buckeridge, a botanist at the Institute of Biosciences at the University of São Paulo (IB-USP).
According to the authors of the study, the new approach could be a simpler alternative to attempting genetic modifications in sugarcane DNA to make its biomass more readily fermentable. The sugarcane genome is complex, with over 100 chromosomes, many of which are repeated, making the creation of viable transgenic varieties challenging. First-generation ethanol production, which accounts for over 98% of the biofuel produced in Brazil, does not require the hydrolysis or saccharification step, as the sugars from sugarcane juice or molasses ferment naturally.
Treatment with these substances has also shown promising results beyond sugarcane: in 90-days-old soybean residues, the compounds increased saccharification by 36%; in Brachiaria grass, commonly used as pasture in cattle farming, the saccharification rate increased by 21% within 40 days of applying the compounds. “The main focus of the experiment was sugarcane, but this approach could also potentially lead to the production of pasture that is more readily digestible, or soybeans with damage-resistant hulls, a desirable trait in the industry,” notes Buckeridge, who led the research team and serves as head of the National Institute for Bioethanol Science and Technology, an organization funded by FAPESP and the Brazilian National Council for Scientific and Technological Development (CNPq). The research team also received funding from the Research Center for Greenhouse Gas Innovation (RCGI), an initiative sponsored by FAPESP and oil and energy giant Shell.
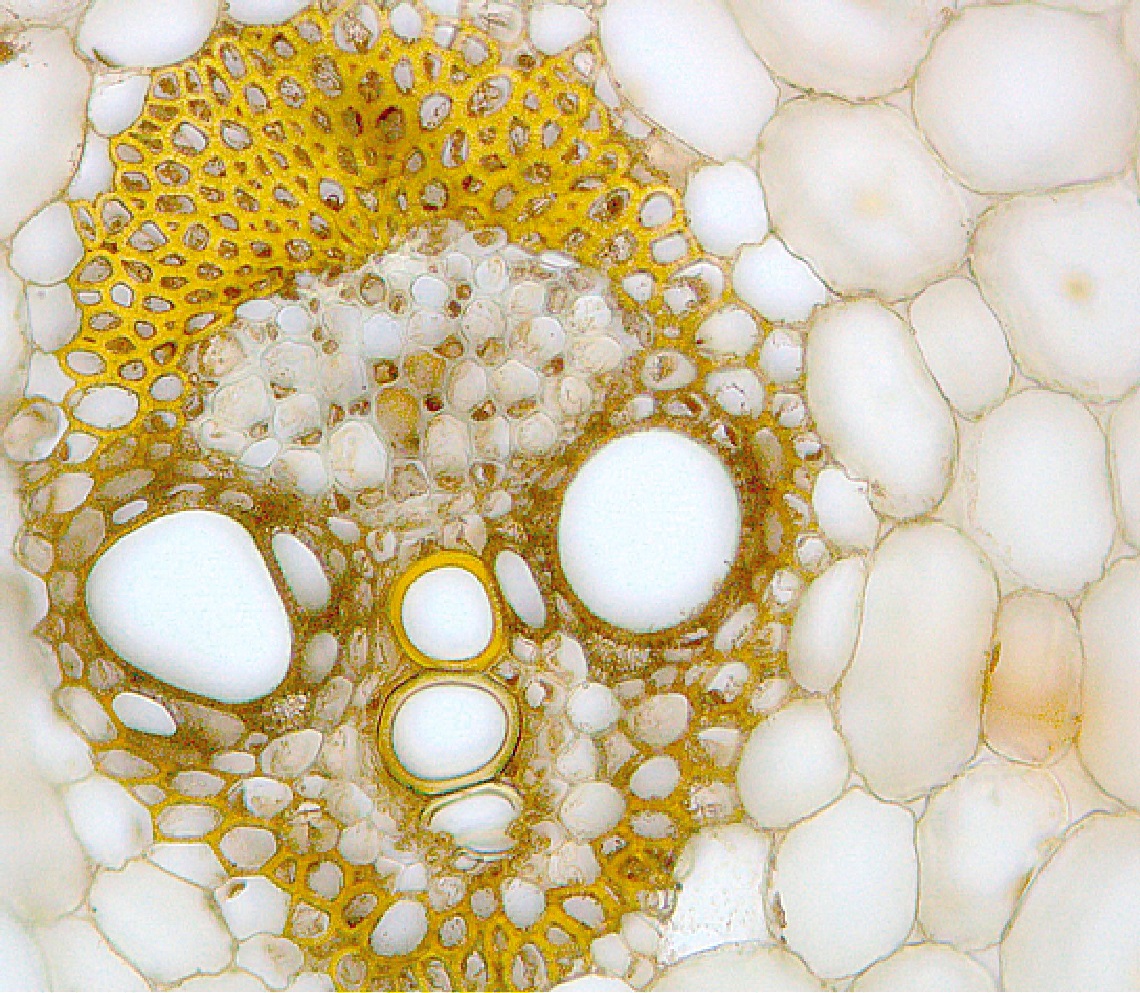
Debora Leite / Marcos BuckeridgeA scanning electron microscope image reveals the presence of lignin (highlighted in yellow) in the cell wall of sugarcane stalksDebora Leite / Marcos Buckeridge
Three compounds that function as inhibitors of enzymes associated with lignin synthesis in sugarcane and other plants were tested: methylenedioxy (cinnamic) acid (MDCA), piperonylic acid (PIP), and daidzin (DZN). Lignin, along with cellulose and hemicellulose, is present in the cell walls of all terrestrial plants, providing mechanical strength, rigidity, and acting as a barrier to the entry of pathogens. Without lignin, plants would not be able to stand upright and would lack an impermeable vascular system to transport water from the roots to the stem.
Different dosages of the compounds were diluted in water and sprayed onto the leaves and roots of the plants at various stages of development. At the end of the experiment, the researchers determined the optimal timing and most effective dosage for applying the substances. PIP proved to be the best-performing compound.
Sugarcane bagasse composition is roughly half cellulose, one quarter hemicellulose, and one quarter lignin. Although essential for plants, lignin creates added challenges in the production of second-generation ethanol. As a non-sugar, it is not fermentable. This, however, is not the biggest challenge. The presence of lignin in sugarcane biomass makes it much more complex and expensive to convert the long carbohydrates in cellulose and hemicellulose — which are not inherently fermentable — into smaller, fermentable sugars. In addition to being difficult to separate from cellulose and hemicellulose in the cell wall, lignin also produces substances that hinder the chemical saccharification process.
Currently, sugarcane bagasse is treated with heat and a combination of enzymes to enable hydrolysis of the biomass. This is the most expensive step in producing second-generation ethanol, accounting for least 30% of the final production cost. “We cannot simply reduce the amount of lignin in a plant as that would be detrimental,” explains Buckeridge. “When we examined the cell wall of sugarcane treated with these compounds under a microscope, we saw no discernible difference. The changes need to be very subtle.”

Wanderley Dantas dos Santos / UEM Sugarcane treated with lignin modulators in MaringáWanderley Dantas dos Santos / UEM
In the article, the researchers note that the use of the compounds did not decrease the overall amount of lignin in the sugarcane bagasse, nor did it alter its composition. However, they noted that the concentration of lignin changed in different tissues of the plant, with a slight increase in the fibrous portion and vessels, and a decrease in the parenchyma, which is a filler tissue in plants. It is possible that this redistribution of lignin in different parts of the sugarcane plant may have contributed to the higher saccharification efficiency reported in the study.
Mario Murakami, a biochemist and the scientific director of the National Biorenewables Laboratory (LNBR) in Campinas, affiliated with the Brazilian Ministry of Science, Technology, and Innovation (MCTI), believes that modulating lignin synthesis using compounds could be a promising approach to potentially enhance the efficiency of second-generation ethanol production. And while he finds the results reported by his colleagues from UEM and USP to be encouraging, he emphasizes the need for validation under industry-relevant conditions. “These experiments need to be conducted under conditions that are closer to those found at plants producing second-generation ethanol to see if real-world saccharification gains match the experimental results and there is no loss of efficiency in the process,” notes Murakami. “The potential impacts of using these compounds on the cost of second-generation ethanol production also need to be determined.”
These questions are expected to be answered soon, as Raízen, one of the two companies producing second-generation ethanol in Brazil (the other being GranBio), is set to begin testing the use of lignin modulator compounds in pilot sugarcane fields later this semester. “Within a year, we may have a clearer picture of whether the saccharification increase is maintained in a commercial operation,” says Santos. Raízen, a joint venture between Shell and the Brazilian group Cosan, is running the experiments in a collaboration with RCGI.
The participating researchers believe that lignin modulators, which can also be synthetically produced in the laboratory, could be developed into a commercial product for the sugarcane and ethanol sector in the future, and possibly for other agricultural applications as well. They have already obtained a patent in Brazil for their use in agricultural crops, and Santos and a group of students from the Department of Biochemistry at UEM have established a startup, BioSolutions, to explore the commercialization of these compounds. Created in 2021, the company is supported by the Brazilian Micro and Small Business Support Service (SEBRAE) in Paraná and the TIM Institute.
The breakdown of sugarcane cell walls into fermentable sugars relies on the action of enzymes
A crucial step in the production of second-generation ethanol is the use of an enzymatic cocktail to hydrolyze cellulose and hemicellulose. Using a combination of enzymes, the complex carbohydrates found in these components of sugarcane bagasse are broken down into smaller, fermentable sugars, such as glucose and xylose. The market for saccharification enzymes is dominated by Danish company Novozymes. “Enzymes represent between 30% and 50% of the final cost of second-generation ethanol production,” says Mario Murakami, a biochemist and scientific director of the National Biorenewables Laboratory (LNBR). As a result, several research groups in Brazil are actively exploring more cost-effective and, ideally, better-performing alternatives to current products.

Maxuel de Oliveira Andrade / Renata rabelo / LNBRSix genes of the fungus Trichoderma reesei were modified to enhance its action in breaking down the cell walls of sugarcaneMaxuel de Oliveira Andrade / Renata rabelo / LNBR
In 2019, Murakami and his team utilized the CRISPR/Cas9 gene editing technique to modify six genes of the fungus Trichoderma reesei, maximizing its production of biotechnologically relevant enzymes, particularly those used for the hydrolysis of plant biomass. Fungi from this genus are commonly employed in the commercial production of enzymatic cocktails designed to promote the saccharification of sugarcane bagasse, enabling the production of second-generation ethanol. In a study published in Bioresource Technology in late 2022, LNBR researchers reported a remarkable reduction in the time taken to produce the enzymes, from 14 to just 9 days. “Our enzymatic cocktail is not only low-cost but also stable, with a one-year shelf life at room temperature,” highlighted Murakami. The cocktail has been successfully tested in the laboratory’s pilot plant and has demonstrated the ability to degrade approximately 60% of the long carbohydrates in sugarcane biomass, a performance similar to some commercial products.
Botanist Marcos Buckeridge from the Institute of Biosciences at the University of São Paulo (IB-USP) suggests that exploring different microorganisms could hold the key to producing enzymatic cocktails for a simpler and more efficient hydrolysis process. In addition to the better-known T. reesei, researchers have also studied fungi found in the Amazon, insects such as cockroaches, and even sugarcane itself as potential sources of enzymes to enhance the process. “The origin of the enzymes is not as crucial,” says Buckeridge, “as mastering the technology for producing an efficient enzymatic cocktail. The high cost of imported products discourages investments in second-generation ethanol.”
Six genes of the fungus Trichoderma reesei were modified to enhance its action in breaking down the cell walls of sugarcane
Projects
1. INCT 2014: National Institute for Bioethanol Science and Technology (nº 14/50884-5); Grant Mechanism Thematic Project; CNPq-INCTs Cooperation; Principal Investigator Marcos Buckeridge (USP); Investment R$5,261,561.47.
2. Research Center for Greenhouse Gas Innovation – RCG2I (nº 20/15230-5) Grant Mechanism Research Centers in Engineering Program; BG E&P Brazil (Shell Group) Cooperation; Principal Investigator Julio Meneghini (USP); Investment R$13,604,936.28.
Scientific article
SANTOS, W. D. et al. Natural lignin modulators improve lignocellulose saccharification of field-grown sugarcane, soybean, and brachiaria. Biomass and Bioenergy. Dec. 5, 2022.


