A lithium-ion battery recycling pilot plant that uses a technique known as flexible hydrometallurgy—a pioneering facility in Brazil—is set to begin operating later this year. The technology was developed by researchers from the Recycling, Waste Treatment, and Extraction Laboratory (LAREX), part of the Department of Chemical Engineering at the Polytechnic School of the University of São Paulo (Poli-USP), in partnership with Tupy, a Brazilian multinational that produces cast-iron structural components.
Hydrometallurgy is a process for separating metals in an aqueous solution, usually with the aid of chemical reagents, carried out at low temperatures. It is an alternative to the traditional battery recycling method based on pyrometallurgy, in which metals are heated to above 1,000 degrees Celsius (ºC), requiring huge amounts of energy and generating more toxic gases as waste.
The use of hydrometallurgy for lithium-ion battery recycling is a recent development. Research centers around the world are refining the technique, but industrial-scale applications remain scarce. The Brazilian pilot plant will be based at the Institute for Technological Research (IPT) in São Paulo.
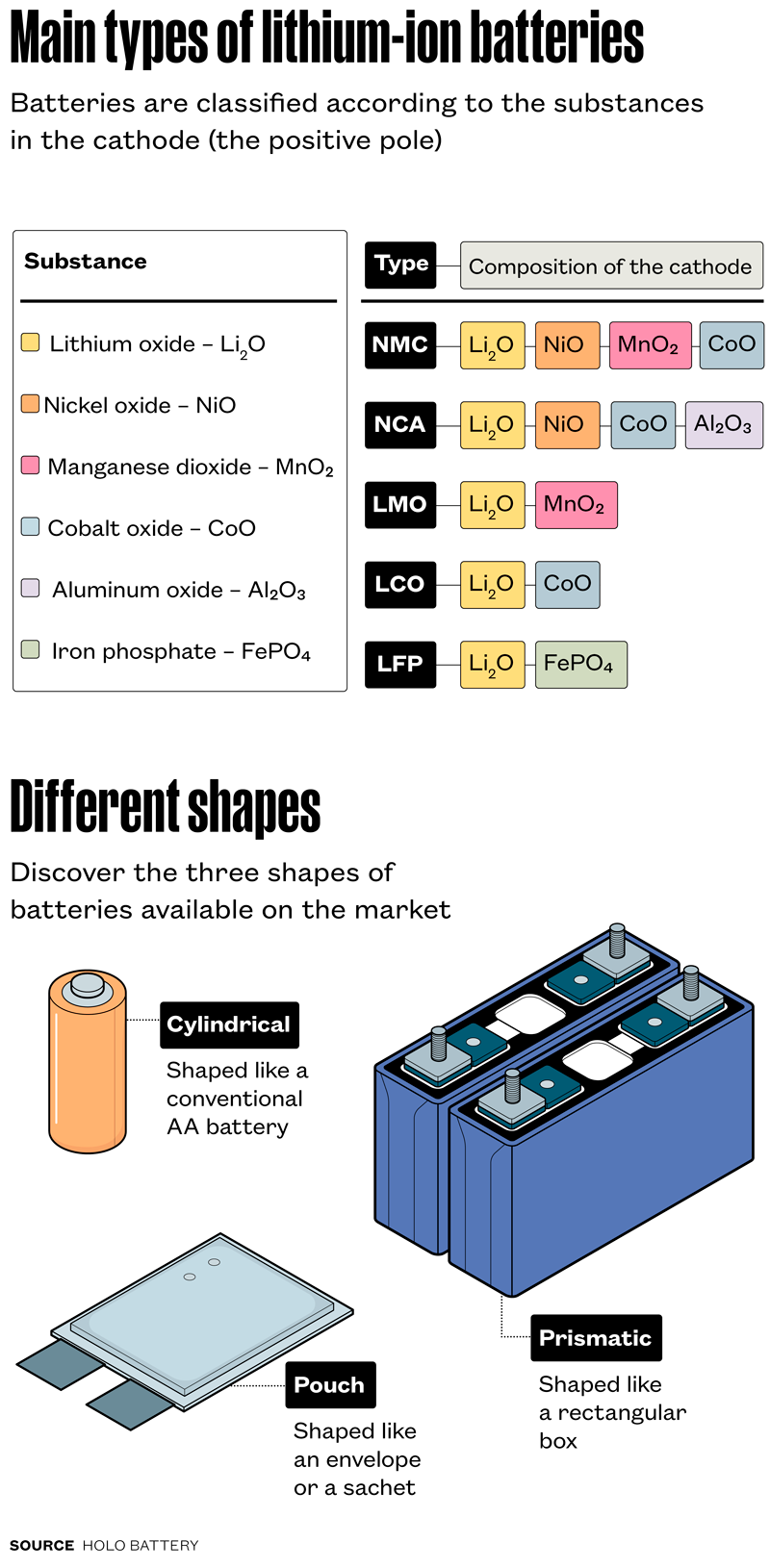
“What makes the technology that will be tested so important is the fact that hydrometallurgy is flexible. In one single process, it can be used to recover several chemical substances that make up the different types of lithium-ion batteries available on the market [see infographic],” says mechanical engineer André Ferrarese, director of research and disruptive development at Tupy. The first studies carried out at USP were funded by FAPESP. Tupy’s project has received funding from the Brazilian Funding Authority for Studies and Projects (FINEP) and the Brazilian Agency for Industrial Research and Innovation (EMBRAPII). Known hydrometallurgical solutions are not universal, since each type of battery has a distinct chemical composition and thus requires its own specific process.
The recycling method begins with disassembly of the battery pack, composed of a set of small battery cells containing an anode (negative pole) and cathode (positive pole) that generate the electrical current. Because these cells degrade at different rates, those still in good condition can be reused in new packs and sold as “second-life” batteries.
Fully degraded cells are subjected to grinding and mechanical or magnetic separation to remove plastics, electronics, and metals such as steel, aluminum, graphite, and copper from their casing. These materials are then sent to their respective existing recycling processes.
In a 2024 article in the scientific journal Industrial & Engineering Chemistry Research, the LAREX team behind the project described an integrated physical processing route for disassembling the three commercial forms of lithium-ion batteries: cylinders, prisms, and pouches.
After grinding and separating the raw materials, what remains is a dark powder known as black mass, formed by a mixture of high-value metal oxides from the anode and cathode. This material, composed of lithium oxides (Li₂O), nickel (NiO), cobalt (CoO), manganese (MnO₂), and other substances, is the primary target for recycling. The hydrometallurgical technique developed at LAREX begins with dissolving the black mass by sulfuric acid leaching, explains metallurgical engineer Professor Jorge Alberto Soares Tenório of Poli-USP, who leads the project together with Professor Denise Espinosa. Leaching is the process of extracting substances from solids through continuous dissolution in an aqueous medium.
The material then passes through a sequence of different reactors, each using a specific mixture of chemical reagents for a particular metal oxide. The selected combination of organic and inorganic reagents, the parameters of each step, and the sequence of reactors make the hydrometallurgical process universal and capable of handling different types of lithium-ion batteries.
The purity level of the materials recovered in the process is around 90%. At this level, the metals can be reused in various industrial applications. However, since lithium-ion batteries require a purity level greater than 99%, explains Ferrarese, reusing recycled materials in new batteries is only possible by mixing them with small amounts of virgin metals.
There are two objectives behind the tests to be carried out at the pilot plant. The first is to scale up production to around 300 liters (L) per recycling batch. In a laboratory setting, the maximum level achieved is 50 L per batch. The second is to test a continuous flow of material in each step of the process, since in the lab, material is transferred between each reactor manually.
The experimental plant will also serve as an industrial demonstration. “Once completed, we intend to commercialize the solution in Brazil and abroad,” says Ferrarese. Tupy’s customers include global manufacturers of machinery, vehicles, and other equipment. Materials recycled at the IPT unit will be used in the country’s first pilot plant for lithium battery production, currently under construction.
Lithium-ion batteries are used in electric vehicles, smartphones, laptops, and other electronic equipment. Estimates suggest that batteries produced with recycled inputs obtained through hydrometallurgy using only inorganic reagents—as in the LAREX process—have a 30% lower carbon footprint than those made entirely with virgin minerals.
Two other lithium-ion battery recycling projects are underway at the Center for Advanced and Sustainable Technologies (CAST) at São Paulo State University (UNESP), São João da Boa Vista campus (see Pesquisa FAPESP issue n° 332). One employs conventional hydrometallurgy focused on LCO (lithium cobalt oxide) and NMC (nickel manganese cobalt) batteries, but uses only organic reagents to reduce environmental impact. The research was funded by EMBRAER and FAPESP’s Research Partnership for Technological Innovation program (PITE).

Léo Ramos Chaves / Pesquisa FAPESPA UNESP researcher holds a lithium battery going through the recycling processLéo Ramos Chaves / Pesquisa FAPESP
According to environmental engineer José Augusto de Oliveira, director of CAST, the research was validated at a pilot unit, achieving a high metal recovery rate of around 90% for lithium oxide, with 98% purity. The work led to a patent filing with Brazil’s National Institute of Industrial Property (INPI) in 2020 and a scientific article published in Resources, Conservation and Recycling in 2021.
The second project at CAST is based on an innovative hydrometallurgy technique using supercritical water (SCW) as a solvent to recover metal oxides. The supercritical state is reached when water is subjected to a temperature above 374 ºC and a pressure of 240 atmospheres (atm).
According to chemical engineer Lúcio Cardozo Filho, a researcher at CAST who is leading the project, preliminary results showed recovery efficiencies above 90% for the metals used in LCO and NMC batteries, as reported in papers published in Chemosphere and Environmental Pollution in 2024.
“The importance of these results has sparked interest in the international scientific community, resulting in collaborations with several groups,” says Cardozo. Partnerships have been established between UNESP and the Royal Melbourne Institute of Technology (RMIT), Australia, and the University of Cádiz, Spain, as well as with other Brazilian institutions, such as the Federal University of Goiás (UFG), the Federal University of Minas Gerais (UFMG), and the University of Campinas (UNICAMP).
A semi-pilot hydrothermal reactor that uses supercritical water to recover critical metals is undergoing final testing at the Laboratory of Supercritical Technology and Phase Equilibria at the Department of Chemical Engineering of the State University of Maringá (UEM), in Paraná. Preliminary tests, Cardozo reports, confirm the laboratory results. If all goes well, the next challenge will be industrial-scale production.
The story above was published with the title “Universal recycling” in issue 354 of August/2025.
Projects
1. Development of innovative processes for recovering critical metals (n° 19/11866-5); Grant Mechanism Regular Research Grant; Principal Investigator Jorge Alberto Soares Tenório (USP); Investment R$3,876,697.58.
2. Sustainable mining: Recovery of critical raw materials from batteries using environmentally sustainable technologies (n° 20/00493-0); Grant Mechanism Regular Research Grant; Principal Investigator Jorge Alberto Soares Tenório (USP); Investment R$122,493.43.
3. Technology for recycling lithium-ion batteries: Lifecycle engineering applications for the circular economy (n° 20/11874-5); Grant Mechanism Research Partnership for Technological Innovation (PITE); Principal Investigator José Augusto de Oliveira (UNESP); Investment R$386,249.22.
Scientific articles
GUILLÉN, D. R. et al. Physical process for Li-ion battery recycling from electric vehicles. Industrial & Engineering Chemistry Research. Nov. 4, 2024.
SANTOS, M. P. et al. A technology for recycling lithium-ion batteries promoting the circular economy: The RecycLib. Resources Conservation and Recycling. Dec. 2021
BARROS, T. V. et al. Assessment of an eco-efficient process for the optimization of metal recovery in lithium cobalt oxide and lithium nickel manganese cobalt oxide batteries. Chemosphere. Sept. 2024.
BARROS, T. V. et al. Recovery of lithium and cobalt from lithium cobalt oxide and lithium nickel manganese cobalt oxide batteries using supercritical water. Environmental Pollution. Oct. 15, 2024.
LIMA, A. M. N. O. et al. Study of the behavior of cations in leaching of NCA lithium-ion batteries by electrodialysis. Environmental Technology. Jan. 29, 2025.
SALES, J. M. A. et al. Precipitation of manganese by ozone from hydrometallurgical recycling process of lithium-ion batteries. Journal of Cleaner Production. Jan. 1, 2024.
CASTRO, R. H. et al. Design of recycling processes for NCA-Type Li-Iin batteries from electric vehicles toward the circular economy. Energy and Fuels. Feb. 29, 2024.
Republish