 AbiuroThe millennia-old practice of using plants to treat disease is blazing new trails in the field of biotechnology. Dozens of experiments by companies and academic institutions all over the world are using techniques to insert genes in the genomes of plants, enabling them to start producing enzymes that have pharmacological value. This will enable transgenic versions of soybean, corn and potato crops, or even ornamental plants, to be used in the future for large-scale production of medical drugs. One example of these experiments in Brazil is currently underway at the Genetic Resources department of the Brazilian Agricultural Research Corporation (Embrapa) in Brasília, Brazil’s federal capital. Researchers there are developing a variety of soybean that produces a viricide or microbicide that can prevent contamination by the AIDS virus. With the help of genetic engineering, these leguminous plants are producing seeds that contain cyanovirin-N, an enzyme whose effectiveness against HIV has already been proven in laboratory tests during pre-clinical trials.
AbiuroThe millennia-old practice of using plants to treat disease is blazing new trails in the field of biotechnology. Dozens of experiments by companies and academic institutions all over the world are using techniques to insert genes in the genomes of plants, enabling them to start producing enzymes that have pharmacological value. This will enable transgenic versions of soybean, corn and potato crops, or even ornamental plants, to be used in the future for large-scale production of medical drugs. One example of these experiments in Brazil is currently underway at the Genetic Resources department of the Brazilian Agricultural Research Corporation (Embrapa) in Brasília, Brazil’s federal capital. Researchers there are developing a variety of soybean that produces a viricide or microbicide that can prevent contamination by the AIDS virus. With the help of genetic engineering, these leguminous plants are producing seeds that contain cyanovirin-N, an enzyme whose effectiveness against HIV has already been proven in laboratory tests during pre-clinical trials.
This type of experiment gained momentum in May 2012 when the United States Food and Drug Administration (FDA) issued its first-ever approval for a pharmaceutical drug produced by genetically engineered plant cells, allowing its commercial use to treat human patients. The active principle of the drug in question is taliglucerase alpha, a protein produced by transgenic carrot cells for the treatment of Gaucher’s disease, a rare genetic condition caused by a lack of glucocerebrosidase, an enzyme our bodies need to process glucocerebrosides, a type of cellular fat. Gaucher’s patients are anemic and have an enlarged spleen and liver. The medication was developed and produced by Protalix, an Israeli company, and is distributed in partnership with US-based Pfizer. It was also approved for sale in Israel and, in March 2013, by the National Health Monitoring Agency (Anvisa) in Brazil, where it will be sold under the name Uplyso. Up until now, Gaucher’s Disease was treated with a different drug, whose active protein is produced by hamster cells modified through a biotechnological process that is more vulnerable to contamination.
The protein synthesized from carrots is similar to the one produced by the human body. For cyanovirin, it’s a different story. The enzyme was isolated in the 1990s in the United States from a cyanobacterium that goes by the scientific name Nostoc ellipsosporum, in research studies by the National Cancer Institute and the National Health Institutes. Cyanobacteria are the blue bacteria commonly and mistakenly called “blue-green algae.” Researchers at NIH and the University of London in England have designed a cyanovirin gel for people to apply before having sexual intercourse. The active principle inhibits replication of the HIV virus by bonding with oligosaccharides (sugars) on the virus’s surface. “Cyanovirin-N is at the pre-clinical development stage, so it hasn’t been tested on humans yet,” says researcher Barry O’Keefe, deputy chief of molecular biology at the NCI’s Molecular Targets Laboratory. O’Keefe spearheaded a study published in 2003, which showed that the protein was also active against some strains of the influenza virus (influenza A and B). He is now taking part in studies to develop cyanovirin. “We still lack a commercially viable, low-cost way to achieve large-scale production of cyanovirin-N, and plants are a good means to that end,” says O’Keefe.
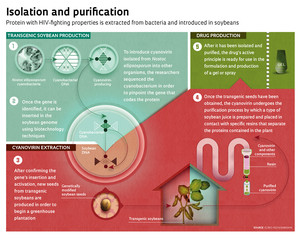 Obtaining large quantities of the protein was the American researchers’ first stumbling block when they had finished the laboratory tests that indicated its effect on some types of virus. The NIH attempted a production method using recombinant DNA, in which the gene that encodes the protein is inserted in the genome of Escherichia coli (which is more easily grown in the laboratory) for subsequent extraction of the desired substance. But productivity was low and it proved to be economically unviable. The solution found by the NIH team, led by O’Keefe, was to seek out Professor Elíbio Rech at Embrapa, the coordinator of the Brazilian group that had filed an international patent for a gene insertion technique for soybeans, and who had experience in developing transgenic crops. “The Americans contacted us in 2007 and we worked out the partnership. They gave us the genetic sequence that encodes the gene, and we inserted it in the genome of Embrapa’s soybean variety 10-16. And it worked. We already have the seeds from the plants we engineered, and they are producing cyanovirin,” says Rech. The researchers isolated the active principle from the soybeans. The viral tests to confirm the effects of the cyanovirin produced by Embrapa were conducted by Professor Amilcar Tanuri at the Federal University of Rio de Janeiro (UFRJ) and by O’Keefe’s laboratory in the US. The result was positive.
Obtaining large quantities of the protein was the American researchers’ first stumbling block when they had finished the laboratory tests that indicated its effect on some types of virus. The NIH attempted a production method using recombinant DNA, in which the gene that encodes the protein is inserted in the genome of Escherichia coli (which is more easily grown in the laboratory) for subsequent extraction of the desired substance. But productivity was low and it proved to be economically unviable. The solution found by the NIH team, led by O’Keefe, was to seek out Professor Elíbio Rech at Embrapa, the coordinator of the Brazilian group that had filed an international patent for a gene insertion technique for soybeans, and who had experience in developing transgenic crops. “The Americans contacted us in 2007 and we worked out the partnership. They gave us the genetic sequence that encodes the gene, and we inserted it in the genome of Embrapa’s soybean variety 10-16. And it worked. We already have the seeds from the plants we engineered, and they are producing cyanovirin,” says Rech. The researchers isolated the active principle from the soybeans. The viral tests to confirm the effects of the cyanovirin produced by Embrapa were conducted by Professor Amilcar Tanuri at the Federal University of Rio de Janeiro (UFRJ) and by O’Keefe’s laboratory in the US. The result was positive.
The latest challenge has been to improve the protein extraction process so as to purify larger quantities of cyanovirin from the soybean seeds. “Our results indicated the presence of 10 grams of the protein per kilogram of fresh seeds. We know that we can’t extract 100% of the drug from the beans because the purification process normally involves a certain level of waste. Up until now, we are up to 20%, or 2g, and our goal is to reach 50%,” says Rech. Purifying the protein is a painstaking, multi-step process. At Embrapa, the researchers are using a resin-based purification method. As the soybean oil undergoes a process similar to filtering, in which the resins serve as filters, the proteins in the soybeans dissolve — including cyanovirin.
“Our intent is to produce a sufficient amount of the protein to test the active principle on macaques in the United States, and later on human beings,” Rech explains. The ultimate goal of the work developed by the NIH, the University of London and the Council for Scientific and Industrial Research (CSIR Biosciences) in South Africa, the groups that are taking part in the research, is to take the gel to Africa where AIDS transmission is still high. Cyanovirin production through tobacco plants is also being tested at the University of London and in the United States. “In tobacco plants, the drug is not just found in the seeds, but is expressed throughout the entire plant. In Africa, under CSIR researcher Rachel Chikwamba, the experiments are also following this same path of cyanovirin production through soybeans and tobacco, but without obtaining success thus far,” says Rech.

EmbrapaSoybean plants genetically modified to produce the cyanovirin protein in an Embrapa greenhouse in BrasíliaEmbrapa
An additional accomplishment by Embrapa in Brasília was the development of a transgenic line of soybeans whose cells produce clotting factor IX, a component of human blood whose absence is one of the causes of hemophilia, a genetic disease that compromises healing and blood clotting, making it difficult to stop hemorrhages. This clotting factor is currently produced either from the blood donated in hospitals or through mouse cell cultures, by inserting the gene that encodes the factor IX protein into the rodent’s genome. “There is also a bottleneck in the development of more efficient and productive purification systems,” says Rech. “We finished this soybean with factor IX in 2012 after five years, we tested the molecule found in the seeds, and now we are transferring the material to the Hemotherapy Center of Ribeirão Preto [of the University of São Paulo (USP)], a partner in the project, to proceed with the molecule purification stage.”
“We received 360 g of freeze-dried transgenic soybeans, and the tests that confirm the presence of factor IX have already been completed. I’ve taken a position as a professor at the Genetics Department of USP’s School of Medicine in Ribeirão Preto, so these studies are now being coordinated by Professors Dimas Tadeu Covas and Lewis Joel Greene at the Hemotherapy Center of Ribeirão Preto,” says biologist Aparecida Maria Fontes, former researcher at the Hemotherapy Center and partner in the soybean research study. “Producing factor IX by using plants is very important because not only does it save blood bank material, which is scarce, but it also creates an alternative using a different production vehicle. To date, the only factor IX molecules made using biotechnology have been produced in hamster cells,” says Fontes.
In all the research studies, and even for future soybean crops genetically modified to produce medicines, a wide range of biosafety initiatives are taken into account. “The plants are grown in contained environments, in greenhouses fully covered by screens. This is done to prevent situations that are actually very unlikely, such as a bird picking up a seed and taking it somewhere else, where it can grow and someone could eat the seeds. It is not poison, but we should treat these plants as a source of medicine, unlike the soybean plants used in food products. Future plantations should also be fenced in, so that no unauthorized personnel will have access,” says Rech.
The advantages of producing drugs in plants include the lower costs introduced by large-scale production, and higher levels of safety when compared with human, fungal, bacterial or animal cells. “It is also easier to manipulate agricultural products. The advantage of soybeans or any other plants is that we can harvest and store them,” says Rech. In an article published in the News in Focus section of the journal Nature in 2012 (May 10), commenting on approval for commercial use of the Gaucher’s disease drug produced from carrots, author Amy Maxmen says that Elelyso — or Uplyso —, the medication approved by the FDA, can be sold for 75% of the price of Cerezyme, the traditionally used drug produced from hamster cells. The traditional treatment can cost up to US$300,000 a year per patient. Maxmen reports that the global market for drugs from biotechnological products achieved the mark of US$149 billion in 2010. “The future of plant-based production methods is very promising for biopharmaceuticals. This is a very exciting time for those who do this type of research,” O’Keefe told Pesquisa FAPESP. “Elíbio Rech and his colleagues at Embrapa are part of a growing industry that very important for the future.”
Scientific articles
O’KEEFE, B.R. et al. Potent Anti-Influenza Activity of Cyanovirin-N and Interactions with Viral Hemagglutinin. Antimicrobial Agents and Chemotherapy. v. 47, n. 8, p. 2.518-25. Aug. 2003.
RECH, E.L. et al. High-efficiency transformation by biolistics of soybean, common bean and cotton transgenic plants. Nature Protocols. v.3, n. 3, p. 410-18. Feb. 2008.

