Intracranial pressure (ICP) monitoring plays a vital role in the management of patients with severe neurological conditions, particularly those requiring intensive care or undergoing neurosurgery for conditions such as hydrocephalus, cranial trauma, stroke, or brain tumors. A research project underway at Universidade Positivo in Curitiba, Paraná, southern Brazil, is working to develop the world’s first invasive sensor capable of transmitting real-time ICP information wirelessly to smartphones and other devices used by medical staff.
“Our new device offers neurologists the benefits of Bluetooth connectivity. It will allow physicians to quickly obtain vital information while providing patients with increased safety and comfort,” explains Erasmo Barros da Silva Junior, an oncological neurosurgeon and the lead author of the study, developed within the Graduate Program in Industrial Biotechnology (PPGBiotec) at Positivo. The project is a collaboration between the Curitiba Institute of Neurology and local companies Orakolo Tecnologia and BMR Medical.
While there are other, noninvasive sensors on the market that can monitor ICP through indirect proxies (see sidebar), only invasive sensors directly implanted in the brain are able to measure cranial pressure in millimeters of mercury (mmHg), the standard unit of pressure measurement. A patient is considered to be in a normal condition when ICP ranges between 5 and 15 mmHg. Readings above 20 mmHg require attention.
“In high-risk patients, particularly those undergoing neurosurgery, an invasive sensor is indispensable,” says Fabiano Moulin, a professor and neurosurgeon in the emergency department and Intensive Care Unit (ICU) at the Federal University of São Paulo (UNIFESP), who has done extensive research on noninvasive methods.
The gold standard method for monitoring ICP involves implanting a small sensor either in the internal chambers (ventricles) of the brain or in the cerebral cortex, the outer layer. The device is wired to bedside equipment where the pressure readings are taken. “Hardwired cables are inconvenient for the patient and also a source of risk. A sudden movement can cause them to disconnect from the monitor. Another disadvantage is that the cables can harbor bacteria and lead to infections,” notes Barros.

Gustavo Benke / Revista Pesquisa FAPESPA prototype of the new sensor: the black case houses the electronic circuitry, Bluetooth system, and battery, while the catheter tube collects the ICP data. The white cable is from a conventional sensor used for comparative measurementsGustavo Benke / Revista Pesquisa FAPESP
The sensor being developed at Universidade Positivo is cable-free, eliminating the risks associated with patient movement. The collected data is transmitted wirelessly to a cloud server accessible via a smartphone or computer application.
The first prototype of the implantable device features two components: a tube with a catheter for collecting ICP data, measuring 35 mm in length by 3.5 mm in diameter, and a square case housing the Bluetooth circuitry. The Bluetooth case, measuring 34 mm on each side and 15 mm in thickness, contains a chipset, a motherboard, and a rechargeable lithium-ion polymer battery.
The new invasive sensor is being developed using fiber optics, which provides a significant advantage over conventional electromechanical sensors currently available on the market. Because optical fiber is immune to electromagnetic interference, the device does not need to be removed and reinstalled for magnetic resonance imaging (MRI) scans, which are common for patients with severe neurological disorders.
Validation in pigs
The new sensor and data-transmission system are currently in the preclinical validation phase, involving animal trials. In the first stage of the research, which concluded earlier this year, the device was implanted in pigs for 120 minutes, and the intracranial pressure readings were compared to those obtained by a conventional hardwired sensor in the same animal. The two sensors showed comparable performance, as reported in a paper in the March issue of the journal Neurosurgery.
A second stage of preclinical research, due to begin shortly, will implant the device in a live animal for a period of 7 to 10 days. This duration is necessary to assess monitoring effectiveness over an extended period of time. Typically, patients with severe neurological conditions require invasive sensors over the space of at least a few days and a maximum of two weeks.
Human trials are the next stage in the research. If successful, Barros expects the wireless sensor to become market-ready within a five-year timeframe. This will require obtaining approval from the Brazilian Health Regulatory Agency (ANVISA).
Two wireless intracranial pressure devices are currently available in the global market. One was developed by the German firm Raumedic AG, and the other by US firm Branchpoint Technologies. However, these devices are not Bluetooth capable and instead rely on a dedicated device that needs to be in close proximity to the patient’s skull to retrieve the data from the implanted sensor. These devices come with a price tag of US$12,000 to US$15,000, according to Barros.
The device being developed at Positivo will now be redesigned for further miniaturization, making it difficult to estimate its commercial cost at this stage. According to Marcelo de Paula Loureiro, a digestive surgeon and professor at PPGBiotec who is serving as Barros’s doctoral advisor, the research team at Positivo University is aiming for a final price that is competitive with cable-connected sensors. These models cost between R$1,000 and R$5,000 per disposable unit.
Loureiro believes the ongoing research on Bluetooth fiber optic sensors for pressure measurement also has potential applications outside neurology. “For instance, the same concept can be used to develop sensors for measuring chest, abdominal, bladder, and ocular pressure,” he explains.
André Giacomelli Leal, a neurosurgeon and chairman-elect of the Brazilian Academy of Neurosurgery (ABNc), believes that the ability to obtain real-time information from neurological patients without the need to physically go to the ICU would be highly advantageous for physicians. “It would save time and make us more proactive. It will also mean we can provide safer care for critically ill patients,” he says.
A brazilian firm developed the world’s first noninvasive intracranial pressure sensor
Launched in 2019, the sensor is expected to reach the international market later this yearThe first noninvasive sensor for monitoring intracranial pressure (ICP) and intracranial compliance (ICC) was developed by Brazilian firm brain4care with grant funding from FAPESP. The device was first introduced on the domestic market in 2019 (see Pesquisa FAPESP issue no. 280), with units now available on a device-as-a-service basis at 50 hospitals and clinics, at a monthly cost of up to R$5,500 per unit.
The brain4care sensor received clearance for commercial use from the United States Food and Drug Administration (FDA) in December 2021, with company management aiming for a commercial launch in 2023. However, the FDA has placed certain restrictions on the device’s use. It cannot be used by individuals under 18 years of age, people with cranial defects, or patients who have undergone surgery involving the removal of part of the cranial bone (decompressive craniectomy or craniotomy).
The sensor, a wearable device attached to the patient’s head with a headband, is connected via the internet to an analytics platform that generates real-time data for display on tablets or other mobile devices. Because the device does not collect pressure data in millimeters of mercury, it is not a replacement for invasive sensors. “Our sensor supports indirect monitoring by sensing minute, nanometric deformations in the skull,” explains Rodrigo Andrade, cofounder and director of research and development at brain4care.
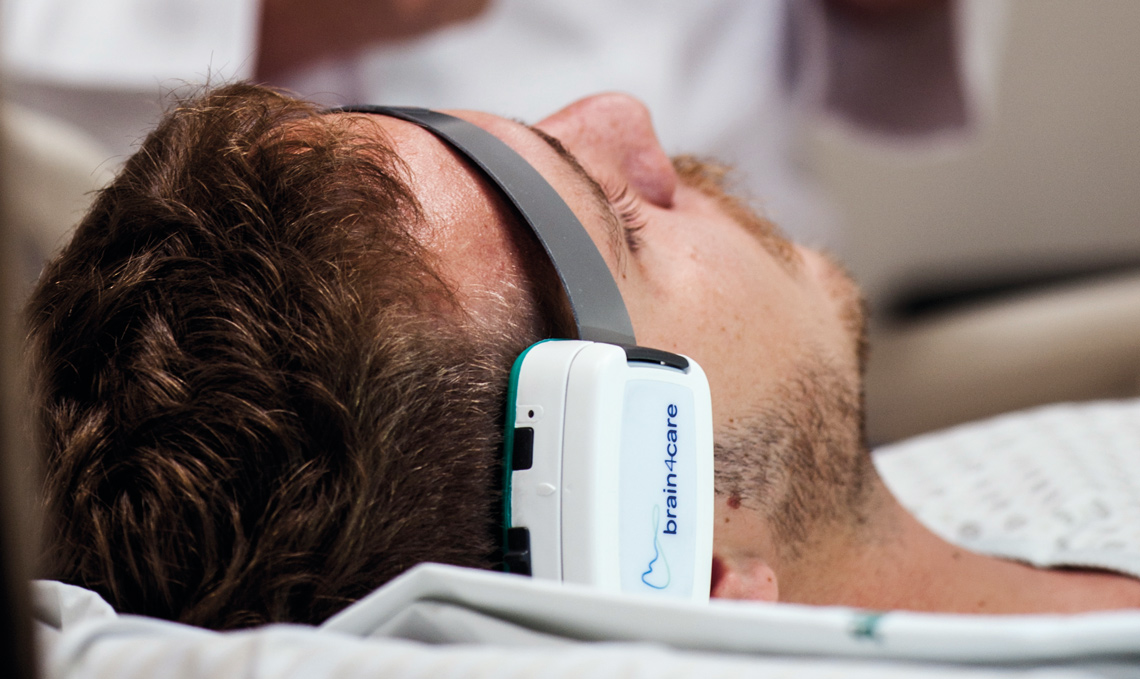
Léo Ramos Chaves / Revista Pesquisa FAPESP The brain4care device is already used in over 50 hospitals in BrazilLéo Ramos Chaves / Revista Pesquisa FAPESP
The device’s noninvasive monitoring method was born out of research by physicist and chemist Sérgio Mascarenhas de Oliveira (1928–2021), a professor at the São Carlos Institute of Physics at the University of São Paulo (IFSC-USP) and one of brain4care’s founders. His research challenged the medical consensus around Monro-Kellie doctrine, a theory formulated in the 1820s by Scottish surgeons Alexander Monro Secundus (1733–1817) and George Kellie (1770–1829). The two surgeons believed that the volume of cerebrospinal fluid, blood, and brain tissue contained in the braincase remained constant.
Mascarenhas, however, observed that the heartbeat generates pressure waves capable of causing deformations in the skull on the order of 5 micrometers (μm). “The deformation of the skull caused by these pressure waves can be used as a proxy for intracranial pressure,” explains Fabiano Moulin, a professor of clinical neurology at the Federal University of São Paulo (UNIFESP), who authored three out of the 59 papers published on Mascarenhas’s observations and noninvasive monitoring of ICP.
The noninvasive method developed by brain4care monitors intracranial pressure by using the ICP waveform to calculate different cerebral hemodynamics parameters. These parameters are deduced from the waveform, pulse by pulse, and used to generate information about intracranial compliance—the skull’s ability to accommodate volume increases without a significant rise in intracranial pressure.
According to Moulin, the diagnostic accuracy rate for ICP is as high as 98%. “It is a highly effective monitoring system for diagnosing low- and medium-risk neurological problems, with the advantage that it does not require surgical procedures to implant a sensor,” he says. According to Andrade, a new miniaturized version of the noninvasive device is currently being developed at brain4care, which the company plans to unveil in 2024.
Projects
1. Development of a minimally invasive device to monitor intracranial pressure (no. 08/53436-2); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Sérgio Mascarenhas Oliveira (Sapra); Investment R$654,281.90.
2. Registration and marketing of a minimally invasive device for monitoring intracranial pressure (no. 11/51080-9); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Sérgio Mascarenhas Oliveira (Sapra); Investment R$348,684.81.
3. Development of a noninvasive sensor, hardware, and software for monitoring intracranial pressure in patients with hydrocephalus and stroke (no. 12/50129-7); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Gustavo Henrique Frigieri Vilela (Sapra); Investment R$358,784.13.
4. Development of a minimally invasive inductive sensor to monitor intracranial pressure (no. 14/50618-3); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Sérgio Mascarenhas Oliveira (Braincare); Investment R$913,895.75.
5. Braincare system for data acquisition, storage, and analysis in healthcare (no. 16/01990-2); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Deusdedit Lineu Spavieri Júnior (Braincare); Investment R$737,309.60.
Scientific articles
SILVA JUNIOR, E. B. et al. Fiber-optic intracranial pressure monitoring system using Wi-Fi — Na in vivo study. Neurosurgery. No. 92(3), pp. 647–56. Mar. 2023.
MORAES, F. M. et al. Waveform morphology as a surrogate for ICP monitoring: A comparison between an invasive and a noninvasive method. Neurocritical Care. No. 37 pp. 219–27. Mar. 2022.
MASCARENHAS, S. et al. The new ICP minimally invasive method shows that the Monro-Kellie doctrine is not valid. Acta Neurochirurgica. Jan. 1, 2012.
Republish


