Chikungunya is a highly debilitating disease. Caused by a virus transmitted by the bite of female mosquitoes of the genus Aedes, it provokes high fever, red rashes across the body, and most notably swelling and severe pain in the joints that can last for months and causes people to walk or remain hunched over. However, few mention the risk of death, which is low but exists, and in certain regions can surpass the national average of deaths from dengue, which is one case in every thousand patients.
Since its arrival to Brazil in 2014, the chikungunya virus has been proven to have infected 254,000 people — suspected cases of dengue have reached 1.2 million — and killed at least 909. Ceará, the most affected state over the 10-year period, has accounted for 31% of the deaths. “We know that chikungunya can kill, but we always wondered: why do the people die?” says Brazilian virologist William Marciel de Souza, of the University of Kentucky, in the USA.
To solve the mystery, Souza and researchers from several institutions in Brazil, the USA, and the UK analyzed blood samples and different tissues from 32 people who died as a result of acute chikungunya infection in 2017 in Ceará. Afterwards, the information was compared with that of 39 individuals who developed milder forms of the disease and survived, and with that of 15 healthy blood donors. The results of the research, which received funding from FAPESP, were published in April in the journal Cell Host & Microbe. The conclusion is that chikungunya kills because the virus, known by the abbreviation CHIKV, spreads across different tissues, including the brain, and causes intense inflammation that damages the organs, preventing them from functioning properly.
Examining the samples, the researchers found that, in general, those who died presented an increased concentration of blood and an accumulation of liquid in the lungs, heart, liver, spleen, kidneys, and brain, despite not having a greater concentration of the virus than the survivors nor being infected with a more aggressive variety of CHIKV.
The blood from those who died also contained significantly higher levels of two groups of chemical communicators than those from the other groups: proinflammatory cytokines, proteins that coordinate the defense, and chemokines, a class of cytokines responsible for attracting cells from the immune system to the sites of inflammation. Associated with symptoms of hyperinflammation, these molecules alter the permeability of the internal wall of the blood vessels, allowing the liquid part of the blood to escape to within the tissues. They also facilitate the penetration of defense cells in the tissues — these cells, while attempting to eliminate the virus, can sometimes destroy healthy cells.
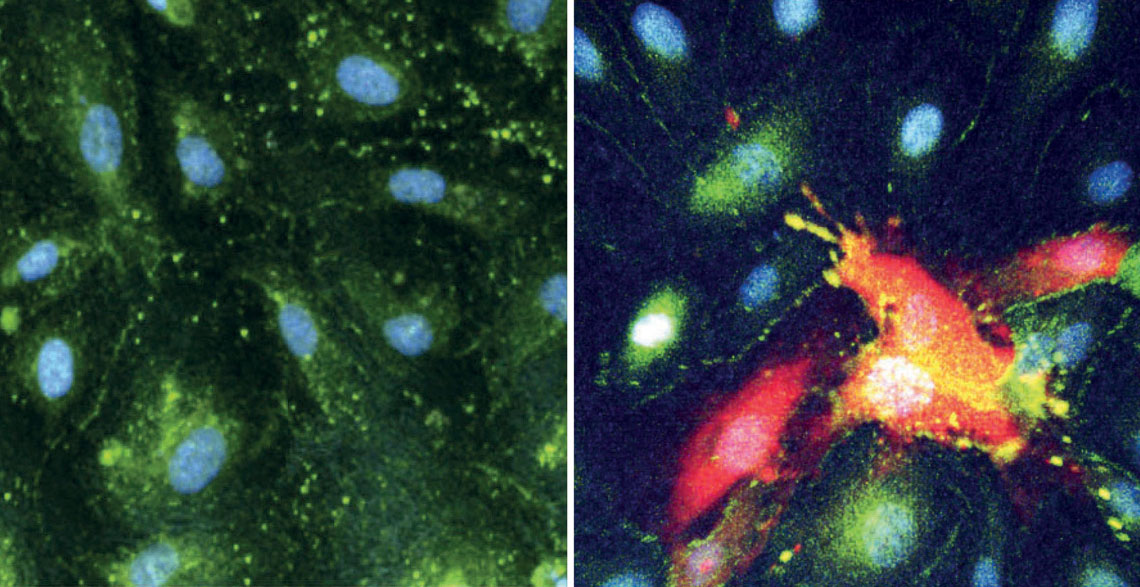
De souza, w. m. cell host & microbe. 2024 Intact cells from the lining of blood vessels of the brain (left), compared to CHIKV-infected cells that have been destroyed (right, in red)De souza, w. m. cell host & microbe. 2024
Among the dead, the presence of defense cells in the heart, liver, kidneys, and, most intriguingly to the researchers, the brain, were observed. The vessels that supply the central nervous system have a special and highly selective lining called the blood-brain barrier. It allows the passage of oxygen, nutrients, and some rare defense cells from the blood to the brain, but usually prevents the entry of pathogens. Souza and colleagues observed that all of those who died had the virus in the cerebrospinal fluid, the liquid that surrounds the brain and other organs of the central nervous system; a sign that CHIKV had crossed the blood-brain barrier. The virus was detected in 13% of brain samples, 20% of heart and kidney samples, 28% of liver samples, 44% of lung samples, and 52% of spleen samples from those who died.
All of the infected people presented metabolic dysregulation, more intense in those who died than in the survivors. This dysregulation affected the integrity and permeability of the blood-brain barrier, potentially facilitating the invasion of the brain by pathogens.
However, this was not the only mechanism. Laboratory tests performed by the group showed that the virus was also capable of infecting the monocytes, defense cells that naturally cross the barrier, using them as a type of Trojan horse. “It hides inside the monocytes and, therefore, reaches the brain,” explains pharmacist Shirlene de Lima, of the Central Laboratory of Public Health of the State of Ceará (LACEN/CE), one of the lead authors of the study.
On analysis of the different organs and tissues affected, the researchers identified extensive damage in the brain with bleeding and cell death. They still do not know which of the factors — hemodynamic imbalance, exacerbated inflammation, or infection of the central nervous system — is most important for defining the fatal outcome. “We need more studies to understand the contribution of each of these problems and why they affect some people more than others,” says Lima. “This knowledge is fundamental for developing better treatment approaches.” Currently, therapy consists of the administration of analgesics, antipyretics, and anti-inflammatories to alleviate the symptoms.
“The study provides relevant information mainly about the behavior of the chemokines and about molecular signatures associated with the patients who died,” states infectologist Julio Croda, of the Oswaldo Cruz Foundation in Mato Grosso do Sul (FIOCRUZ-MS), who did not take part in the study. “The infiltration of the infected monocytes in the brain and its effect is a new finding. Now we need larger studies, with people of different ethnicities, ages, and genders, to validate these conclusions.”
Until a more effective treatment arrives, hope rests on the arrival of a vaccine. In November 2023, the Food and Drug Administration (FDA), the agency that regulates food and drugs in the USA, approved the use of Ixchiq for adults, a vaccine based on the weakened virus developed by Franco-Austrian pharmaceutical company Valneva. In Brazil, the company has a partnership with the Butantan Institute, which is currently testing the composition in phase 3 clinical trials in adolescents before submitting the request for approval by the Brazilian Health Regulatory Agency (ANVISA).
Projects
1. Multiomic approaches to the study of Chikungunya disease (nº 19/24251-9); Grant Mechanism Postdoctoral Fellowship Abroad; Supervisor Luiz Tadeu Moraes Figueiredo (USP); Beneficiary William Marciel de Souza; Investment R$301,943.10.
2. Characterization, genomics, and diagnosis of viruses with public health importance in Brazil by high-performance sequencing (nº 17/13981-0); Grant Mechanism Postdoctoral Fellowship; Supervisor Luiz Tadeu Moraes Figueiredo (USP); Beneficiary William Marciel de Souza; Investment R$303,193.93.
Scientific article
DE SOUZA, W. M. et al. Pathophysiology of chikungunya virus infection associated with fatal outcomes. Cell Host & Microbe. Apr. 2024.
