An article published in the journal Nature Medicine in January presented five human cases of a potentially new and very rare form of Alzheimer’s disease known as iatrogenic Alzheimer’s, acquired through medical procedures. The newly discovered form of the disease only occurs in exceptional circumstances.
The five patients were treated by specialist services in the UK health system between 2017 and 2022, having been referred by other hospitals. They presented typical symptoms of the disease, such as speech and memory problems and difficulties planning routines. Two had previously received a clinical diagnosis of Alzheimer’s supported by markers in blood tests or imaging exams, and a third case was confirmed at autopsy, which identified brain lesions characteristic of the disease.
The patients, seen by a team led by neurologist and study leader John Collinge of University College London (UCL), were all younger than usual. They were between 38 and 55 years of age when they began showing symptoms, but the sporadic form of the disease—which accounts for almost 99% of cases—tends to appear after the age of 65. Neither they nor their parents had the gene mutations associated with hereditary Alzheimer’s, which is responsible for around 1% of cases.
All, however, shared something in common. In childhood, they suffered stature problems and were treated with intramuscular injections of a growth hormone extracted from the pituitary gland of deceased donors. After evidence emerged that use of the hormone could lead to the development of a fatal neurological illness called Creutzfeldt-Jakob disease (caused by an infectious protein called a prion), a synthetic version began being used worldwide in 1985.
Collinge is head of UCL’s Department of Neurodegenerative Disease and the British national health system’s National Prion Clinic, which specializes in diagnosing and monitoring people with illnesses caused by the infectious protein. Prions are defective versions of a protein that is abundant on the surface of neurons and plays an important role in how they function, responsible for processing information in the brain. They can emerge spontaneously or they can be acquired through the consumption of infected beef or the use of contaminated neurosurgical instruments and tissue implants (see Pesquisa FAPESP issue nº 148). In the central nervous system, they induce deformation of healthy proteins, which become toxic and start killing neurons, leaving the brain porous like a sponge.
“Our findings suggest that Alzheimer’s and certain other neurological diseases share similar evolutionary mechanisms to Creutzfeldt-Jakob disease,” Collinge said in a press release.
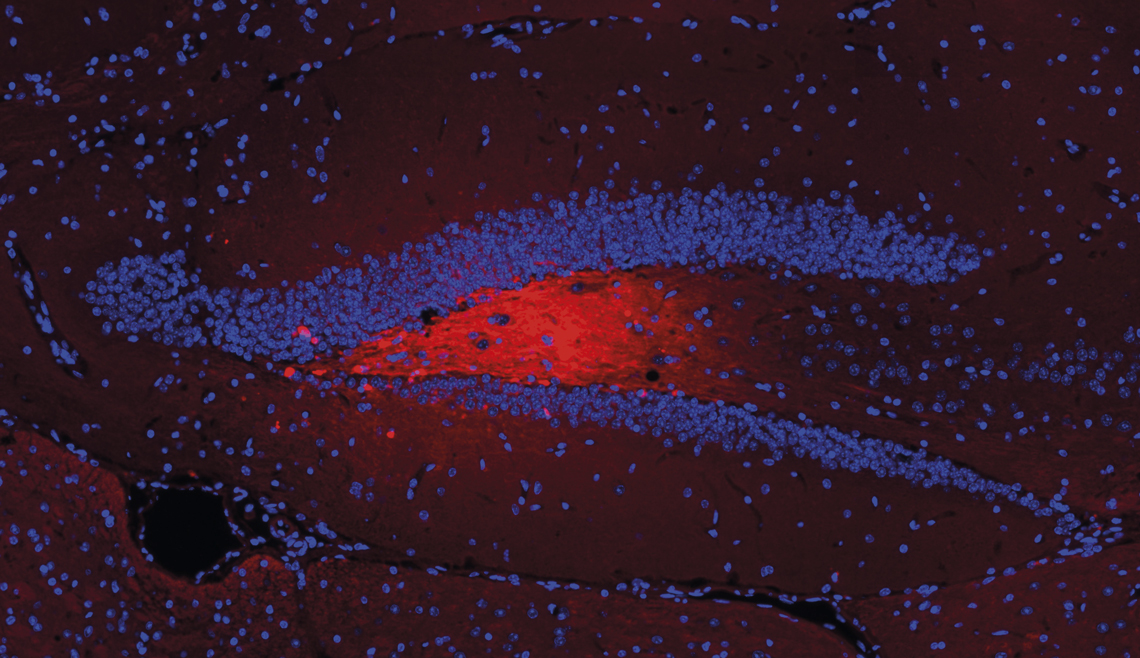
Victor Bodart Santos / UFRJThe image above shows vesicles (red) containing amyloid beta injected into the hippocampus (blue V shape) of miceVictor Bodart Santos / UFRJ
Alzheimer’s is the most common form of dementia in the world, responsible for up to 70% of all cases. It begins with the accumulation of fragments of amyloid precursor protein on the surface of neurons. As people age, they begin to process this protein abnormally and break it down into fragments called amyloid beta peptides, which can form clumps. If too many of these peptides are produced or not enough are eliminated, they accumulate and form plaques on the outside of neurons and trigger harmful events, causing tau proteins in a hyperphosphorylated state to cluster together into toxic tangles over time. These tangles then kill the neurons, resulting in the clinical symptoms of the disease. In recent years, it has been shown that amyloid beta peptides and hyperphosphorylated tau proteins can spread between neurons through a similar process to prions.
Collinge and his colleagues had suspected for over a decade that like prions, amyloid beta peptides can be transmitted from one person to another, initiating the “seeding” of the disease. The first evidence emerged when UCL neuropathologist Zane Jaunmuktane identified amyloid beta clumps in the brains and blood vessels of the central nervous systems of four people who developed Creutzfeldt-Jakob disease after being treated with growth hormone that was contaminated with prion. However, the results, published in Nature in 2015, did not show whether the amyloid beta plaques were there before the prions or whether they had been seeded by them.
Suspicion that the peptides are transmissible grew in 2018. Silvia Purro, a chemist from the UCL team, analyzed batches of growth hormone administered to the Creutzfeldt-Jakob patients and found that in addition to prions, they contained amyloid beta and hyperphosphorylated tau proteins. Purro injected the contaminated samples into the brains of mice, showing in another Nature article that they had seeded the peptides and caused Alzheimer’s lesions. All that remained was to prove that the same could have happened to people given the hormone during their childhood.
The Nature Medicine article provided this confirmation. Some of the Alzheimer’s patients had received hormones from batches containing amyloid beta and tau proteins, including the batches that proved capable of seeding the disease in rodents. “We have found that it is possible for amyloid beta pathology to be transmitted and contribute to the development of Alzheimer’s disease,” said neurologist Gargi Banerjee, lead author of the study, in a press release.
“However, it is a rare occurrence. It was transmitted via a very specific route. It is not a contagious agent that can pass from one person to another through mere contact or exchanging bodily fluids, for example,” emphasizes neurologist Adalberto Studart Neto, a member of the Cognitive and Behavioral Neurology Group at the University of São Paulo School of Medicine (FM-USP) who did not participate in the study. Of the 1,848 people treated with the growth hormone from deceased donors in the UK between 1959 and 1985, only five (0.3%) were contaminated with the peptide and developed Alzheimer’s as a result.
In a comment published in the same issue of Nature Medicine, Swiss neuroscientist Mathias Jucker, from the University of Tübingen, Germany, and American neuroscientist Lary Walker, from Emory University, USA, remained cautious. “On the one hand, it is prudent to consider these conclusions with a measure of skepticism. The cases presented are diverse and complicated; the individuals had undergone a variety of medical interventions for various disorders earlier in life, and it is difficult to exclude a contribution of these circumstances to the complex disease phenotypes that appeared many years later,” they wrote. “On the other hand,” they continued, “there is good reason to take these findings seriously. Only individuals who received cadaveric growth hormone prepared in a specific way developed the features of Alzheimer’s disease.”
At around the same time as evidence was emerging that these components are transmissible, researchers—including Brazilians—were demonstrating the importance of a mechanism that helps the disease spread in the brain: the production of extracellular vesicles, which are small sacs (the size of a virus) containing proteins, genetic material, and even organelles.
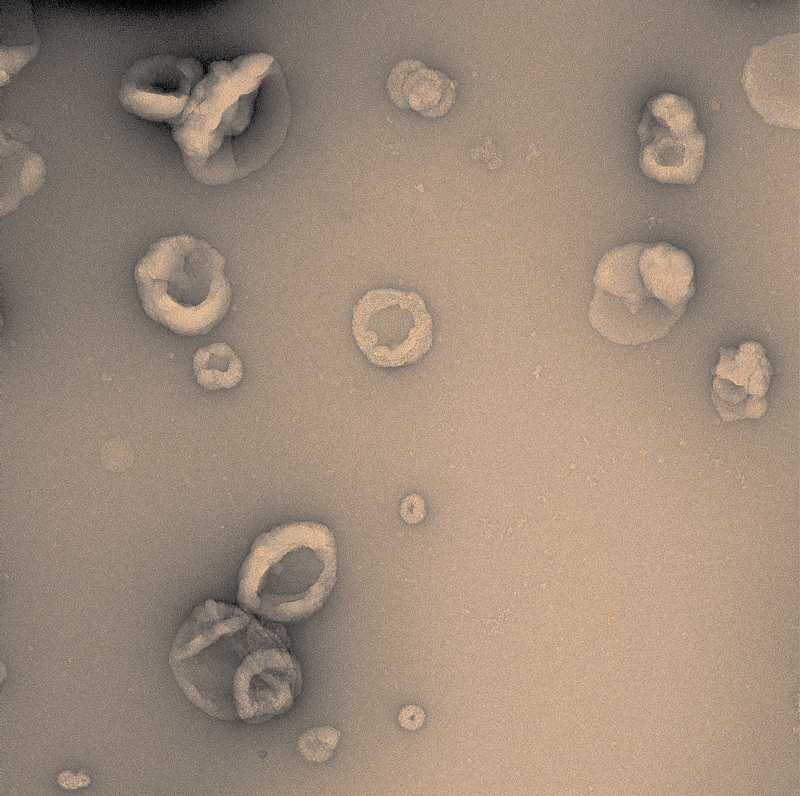
Victor Bodart Santos / UFRJVirus-sized vesicles carry proteins and help spread lesions associated with Alzheimer’sVictor Bodart Santos / UFRJ
“For a long time, they were considered the garbage truck of cells, used to get rid of parts that no longer worked,” says biochemist and neuroscientist Fernanda De Felice of the Federal University of Rio de Janeiro (UFRJ), who investigates the role these cell structures play in Alzheimer’s.
The idea that these small sacs could serve other functions began to arise when it was observed that in certain situations, they act as messengers between cells, in addition to contributing to the spread of Alzheimer’s lesions. In an article published in the Journal of Biological Chemistry in 2012, a group led by neuroscientist Efrat Levy of New York University isolated vesicles produced in the brains of rodents used to study Alzheimer’s and showed that they could carry copies of the amyloid beta peptide.
Later, neuroscientist Tsuneya Ikezu and his team from Boston University found that vesicles obtained from the brains of people with Alzheimer’s contained much more amyloid beta and hyperphosphorylated tau proteins than those from healthy individuals, according to an article published in the journal Alzheimer’s & Dementia. In another paper, published in Brain in 2021, the Boston group found that vesicles containing tau proteins could spread Alzheimer’s lesions in the brains of rodents.
“In these situations, they are like a Trojan horse,” says Brazilian neuroscientist Victor Bodart Santos, who is currently on a postdoctoral fellowship at Ikezu’s laboratory at the Mayo Clinic, USA.
In research carried out as part of his PhD, supervised by De Felice and described in Alzheimer’s & Dementia in 2023, Bodart Santos proved that as well as spreading the lesions, vesicles loaded with amyloid beta and tau proteins induce clinical signs of the disease.
He injected vesicles containing these components into the brains of rodents and subjected them to a series of tests. In experiments designed to assess their motivation to explore their environment, ability to locate a shelter, and ability to recognize objects, the animals performed much worse than others that were given vesicles without amyloid beta and tau proteins. The damage was more severe and appeared earlier when the rodents were genetically altered to present the human version of the tau protein.
“Although vesicles are not seen as the primary mechanism for spreading the disease in the brain, this research confirms that alone they may be sufficient to cause Alzheimer’s,” says neurologist Wyllians Borelli, head of research at the Moinhos de Vento Hospital Memory Center in Porto Alegre, Rio Grande do Sul.
“Vesicles seem to be an efficient way of disseminating the components of the disease in the brain because they protect them from the deterioration they might suffer in the extracellular environment,” says Bodart Santos. “They were able not only to propagate the lesions but also to make the animals demonstrate behaviors typical of the disease,” points out De Felice.
Scientific articles
BANERJEE, G. et al. Iatrogenic Alzheimer’s disease in recipients of cadaveric pituitary-derived growth hormone. Nature Medicine. Jan. 29, 2024.
JAUNMUKTANE, Z. et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature. Sept. 10, 2015.
PURRO, S. A. et al. Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature. Dec. 13, 2018.
JUCKER, M. & WALKER, L. C. Evidence for iatrogenic transmission of Alzheimer’s disease. Nature Medicine. Jan. 29, 2024.
JAUNMUKTANE, Z. et al. Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathologica. Feb. 15, 2018.
PEREZ-GONZALES, R. et al. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. Journal of Biological Chemistry. Dec. 2012.
MURAOKA, S. et al. Proteomic and biological profiling of extracellular vesicles from Alzheimer’s disease human brain tissues. Alzheimer’s & Dementia. Apr. 17, 2020.
RUAN, Z. et al. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain. Feb. 12, 2021.
BODART-SANTOS, V. et al. Alzheimer’s disease brain-derived extracellular vesicles reveal altered synapse-related proteome and induce cognitive impairment in mice. Alzheimer’s & Dementia. May 19, 2023.
