Shortly after 11 a.m. on April 10, veterinarian and embryologist Ligiane Leme stood in a dark room, looking closely at a computer screen. The monitor, the only source of light in the room, showed an image of an immature egg, held by a micropipette near a tiny needle. In the following moments, fellow embryologist Georgina Hastenreiter used a joystick to move the needle, making a minuscule puncture in the cell before using a series of quick and precise movements to extract its genetic material. The nucleus-less egg was then filled with DNA from an adult pig skin cell.
Leme, Hastenreiter, and biomedical scientist Tainah Moraes spent the rest of that day and the next repeating the procedure on another 600 eggs, collected at a slaughterhouse in the interior of São Paulo. The pigs’ reproductive cells, conditioned in a culture medium, were taken to the FAPESP-funded Human Genome and Stem Cell Research Center (HUG-CELL) at the University of São Paulo (USP), where the next stage of the ambitious project would take place: an attempt to produce clones of genetically modified animals in Brazil, to provide organs for human beings and potentially help reduce transplant waiting lists. According to data from Brazil’s Ministry of Health, 71,000 people were awaiting an organ or tissue donor in April.
“Brazil has the largest public organ transplant system in the world,” points out Ernesto Goulart, a pharmacist who specializes in tissue bioengineering and heads the project at USP. “We believe we have an obligation to develop a strategy that allows this system to continue functioning well and serving more people,” says the researcher.
The project is coordinated by HUG-CELL geneticist Mayana Zatz and surgeon Silvano Raia, from USP’s School of Medicine (FM). It received early funding from the pharmaceutical company EMS and is now funded by FAPESP and the federal government. It currently involves almost 50 researchers from USP, the Brazilian Agricultural Research Corporation (EMBRAPA), the Federal University of São Paulo (UNIFESP), and the Institute for Technological Research (IPT).
“Organ transplantation was the greatest advance in the history of surgery. Between 2000 and 2022, two million transplants were carried out worldwide. Since this is a life-saving procedure, that means two million lives saved,” emphasizes Raia. “Due to its success, demand now exceeds supply. We therefore need to find additional sources for organs,” says the surgeon, one of the world’s pioneers of liver transplants from living donors.
At the end of those two days in April, the team of scientists had obtained 280 cloned embryos, which they then transferred to the uterus of two female hybrids of the landrace and large white breeds. If everything goes as planned, they will know by mid-May whether the sows are pregnant and how many offspring they are expecting. The hope is that after almost four months of gestation, at least one piglet will be born.
When the cloning is successful, we want to be ready to test the edited cells, says Goulart, from USP
There is great expectation, despite the low chance of success. Only a small fraction of the cloned embryos transferred to the recipient females successfully implant in the uterus, and of these, an even smaller proportion (between 1% and 5%) reach full term and are born.
In the last year, Leme’s team has already produced more than 10,000 cloned pig embryos and carried out 20 transfers to recipients, inserting around 200 embryos at a time. The females have become pregnant every time, and in some cases, the fetuses developed for up to 50 days, almost half the length of a pig’s pregnancy. But to date, none of the pregnancies have resulted in the birth of a piglet, something the researchers hope will soon change, thanks to improvements made with each new attempt. “In Brazil, cattle cloning has been done for some time, but the technology is still incipient when it comes to pigs,” says Goulart. “The technique has been less used with pigs due to the characteristics of the species’ gametes,” explains Leme.
The challenging nature of the task is not limited to Brazil. Approximately 15 groups in at least eight countries are trying to clone genetically modified pigs to obtain organs, but only three have succeeded. One was the American pharmaceutical company eGenesis, which supplied the pig kidney transplanted to a man with kidney failure in March.
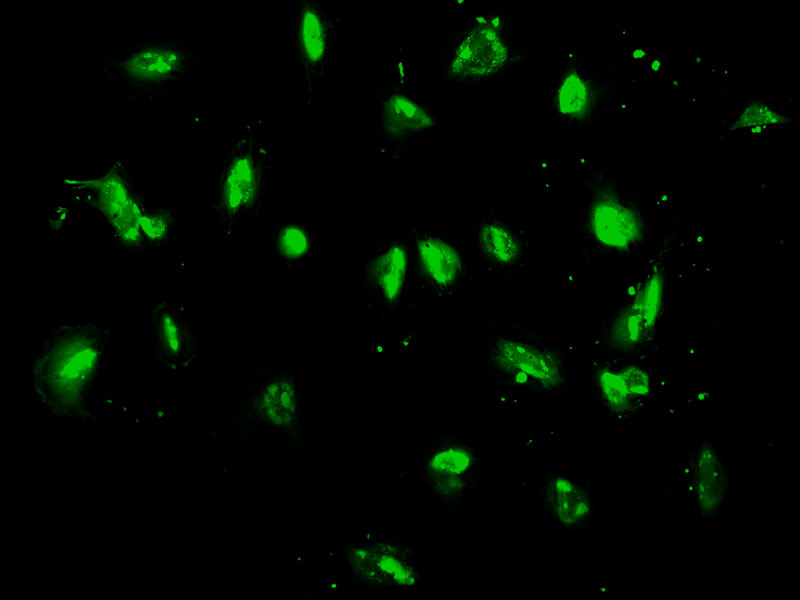
Tulio Yoshinaga / Cegh-Cel / USPGenetically modified skin cellsTulio Yoshinaga / Cegh-Cel / USP
There are innumerable factors behind a clone’s success rate: the temperature at which the cells are handled, the amount of light (which is why the room is dark), the synchronization between the division stage of the DNA donor cell and the egg, the phase of the reproductive cycle at which the embryos are transferred to the recipient, and the number of embryos actually implanted in the uterus. Pigs have 20 to 25 piglets per litter, and if the number of embryos is much smaller, the pregnancy will not continue. In April, the USP team turned to Brazilian veterinarian and embryologist Luis Queiroz, who helped develop the eGenesis animals, to help them improve their cloning process.
Successfully cloning their first pigs would confirm that the USP group has finally mastered the technique, an essential step alongside another process needed to make animal organs more compatible with the human body: genetic cell editing. Despite the genetic differences and other incompatibilities, pigs have been the donor animals of choice in recent years because their organs are similar in size and function to those of humans, in addition to being domesticated animals that reproduce well in captivity and produce large litters after short pregnancies.
Five years ago, before the cloning project, together with biologists Luiz Caires and Luciano Abreu Brito, Goulart began tests on altering the genetic configuration of porcine cells to reduce one of the main risks of transplantation: rejection. Resulting from an attack on the new organ by the recipient’s immune system, rejection can also occur when the donors are human beings. It is generally more common, however, with organs from other animals, which is known as xenotransplantation.
At the HUG-CELL’s Gene Editing Laboratory, just down the hall from the cloning room, Goulart, Brito, and colleagues are using two techniques to manipulate the DNA of cells to be used to make clones. One is the CRISPR-Cas9 gene-editing tool (see Pesquisa FAPESP issue nº 288), with which the USP team was able to inactivate three genes that are normally switched off in xenotransplantation experiments: GGTA1, CMHA, and B4GALNT2. These genes encode enzymes involved in the production of sugars on the surface of porcine cells that are recognized by the human immune system and trigger hyperacute rejection, during which antibodies present in the recipient’s blood destroy the organ within hours or days of the transplant.
More recently, the group began using a second technique — the PiggyBac (PB) transposon system, which uses a stretch of DNA capable of cutting the double strand of cellular DNA and inserting new segments — to add seven human genes to porcine cells. The objective is to create cells, and consequently embryos and organs, with more human-like characteristics, to circumvent the immune system and avoid the production of new antibodies, which can cause acute rejection months after the transplant. “The entire gene-editing process, especially the addition of genes, is delicate. It represents an attack on the cell,” explains Brito.
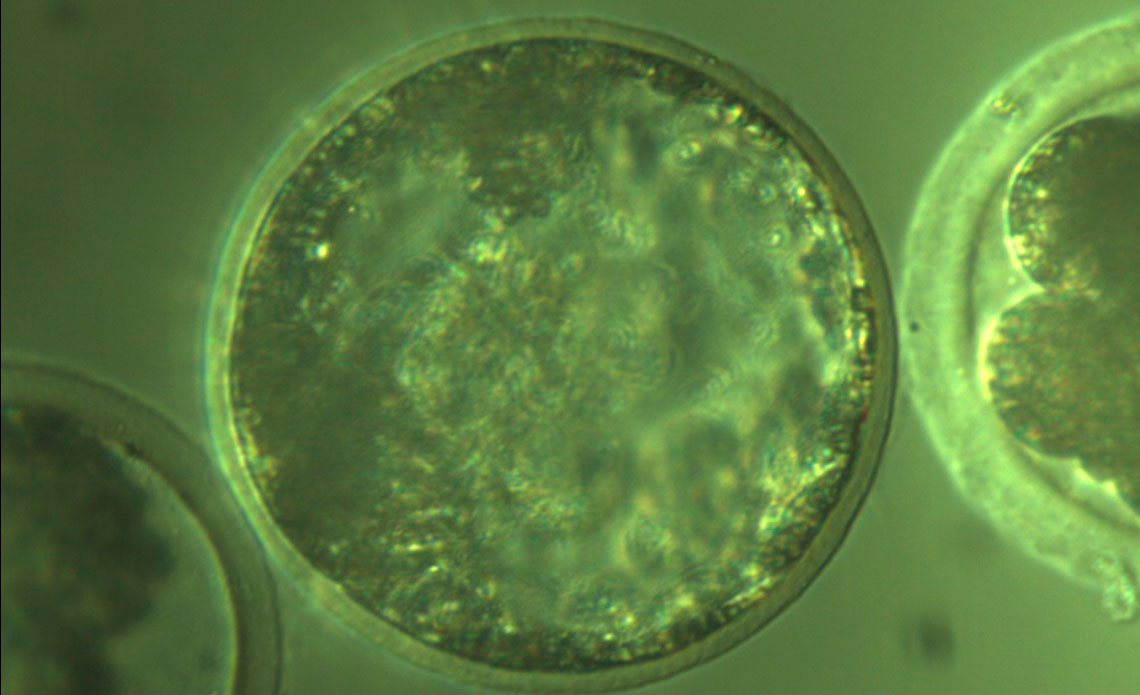
Ligiane Leme / Cegh-Cel / USPA cloned pig embryoLigiane Leme / Cegh-Cel / USP
By April, the scientists had successfully inserted the genes into fibroblasts, the skin cells most suitable for cloning, but they were yet to determine whether they had been incorporated into the desired stretch of the genome or whether they enabled adequate production of the proteins they codify. The team now plans to sequence the DNA of the fibroblasts, to identify the insertion point of the genes.
“When the cloning is successful, we want to be ready to test the edited cells,” says Goulart, who hopes they will be able to produce kidneys, corneas, hearts, and skin for xenotransplantation. The cloned pigs and their offspring will be kept in two special facilities designed for breeding animals that will donate organs to humans. The first, with capacity for up to 10 animals, was opened on USP’s São Paulo campus on April 23. Another larger one is under construction at the IPT. After they are born, the animals will be cared for by XenoBR, a startup created from the project that will provide the organs for clinical tests.
Advances in gene editing over the last decade and the potential for reducing the risk of rejection has revived medical interest in organs of animal origin — a long-time pursuit that has been documented since at least the seventeenth century (see timeline below). Since 2022, at least three critically ill patients have received a porcine organ transplant as a final option (compassionate treatment).

