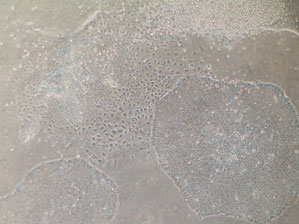
Laboratory of Lygia da Veiga Pereira/ USPLive colony: embryonic stem cells multiply on a plate that has an artificial environmentLaboratory of Lygia da Veiga Pereira/ USP
Stem cells removed from human embryos have been heralded as the great hope for curing many diseases against which the hands of today’s medicine are to a certain extent tied. Such is the case of certain cardiac problems, genetically-originated anomalies, like muscular dystrophy and degenerative diseases of the nervous system, like Parkinson’s disease. Constantly appearing on the science and health pages of newspapers and journals these cells that can give rise to any human tissue, have gained even more prominence since October 2 when geneticist Lygia da Veiga Pereira, from the University of São Paulo (USP), announced she had obtained a Brazilian strain of embryonic stem cells – the BR-1.
The news was celebrated by colleagues who were present at the III International Cell Therapy Symposium in Curitiba, Paraná. After the presentation some researchers had suggestions of how to test the capabilities of the cells. “But what they really wanted was to know when the cells would be available”, says Lygia. Even those who did not follow this line say that the achievement is important and gives a degree of independence to Brazilian researchers. Over and above substituting the importance of this type of cell the geneticist from USP is celebrating the technical competence that her group showed in obtaining and maintaining the strain. She began working with imported embryonic stem cells immediately after the passing of the Biosafety Law in 2005, which regulates this type of research. In 2006 she brought in foreign researchers, like Prithi Rajan, from the Burnham Institute in San Diego, in the United States, to show how to make the cell culture. “She taught us the ideal culture conditions and showed how to train the eye to see when the cells are ‘happy'”, she recalls. Even so the first strains died. “We had to change some details in the culture environment and when we did so we showed that we could do it alone.” Cardiologist José Eduardo Krieger, from USP’s Heart Institute (InCor) agrees: “The most important thing is mastering the technology. That’s the only way we’ll be able to actively interfere in the whole research process with cell therapy. You can’t put a price on that.” The president of the Federation of Experimental Biology Societies (Fesbe), Luiz Eugênio Mello, adds: “In many American strains it’s difficult to know if the cells have been kept in good condition. It’s different when you have a neighbor who can give you all the characteristics of the material you’re working with”.
Another advantage is agility in getting hold of cell samples. For geneticist Mayana Zatz, from the Human Genome Studies’ Center at USP, the great obstacle to carrying out research with stem cells in Brazil is the time spent importing all the material. “It takes months for us to receive reagents that Americans can get hold of less than 48 hours”, she says. “In a competitive area like this it becomes almost impossible for us to publish in international periodicals.”
Despite being celebrated, the investment necessary for developing a strain of embryonic stem cells is subject to criticism for being more a technical advance than a scientific one. Lygia is the first to admit: “There’s no scientific innovation in this result; this is the spade work that someone had to do”. Obtaining stem cells consists in removing around 50 cells from a five day old embryo when it is only a mass of just 150 cells, and making sure they stay alive and divide, but without changing into specific tissue cells, like skin, muscle or the nervous system. This capacity to give rise to so many cell varieties – pluripotency – makes embryonic stem cells the organism’s wild card.
There is no need for any very special installations to work with these cells: Lygia set up her Molecular Genetics Laboratory with the help of FAPESP in previous projects. In addition to basic equipment, like reagents and microscopes, all that is needed is to have an isolated area for dealing with the cells and an incubator for keeping them at a comfortable temperature of 37o Celsius. It seems simple, but few laboratories in Brazil have the formula for keeping the cells under these conditions. Even so the feat of Lygia’s group is nothing new, because stem cell strains already exist in various countries – the pioneer was North American James Thomson, from the University of Wisconsin, in 1998.

Photos Laboratory of Lygia da Veiga Pereira/ USP
A five day embryo from which pluripotent cells are removedPhotos Laboratory of Lygia da Veiga Pereira/ USPKnowledge transferred
Obtaining a strain of embryonic stem cells is above all proof of the patience and great persistence needed to find the exact composition of the liquid that nurtures the cells. For this Lygia can count on a team of students, some of whom have FAPESP scholarships, although the specific project that led to the BR-1 has been funded by the National Council for Scientific and Technological Development (CNPq) and by the Ministry of Health. To oblige the cells to remain pluripotent, the traditional procedure was to cultivate them on a layer of mouse fibroblasts. These cells secrete a combination of substances that maintain the culture in this versatile state, but researchers are concerned because they also produce typical rodent compounds that may contaminate the human cells that would become unsuitable for therapeutic purposes. Lygia tried using human fibroblasts but was not successful. Finally, last year, she resorted to a new tool: an artificial culture environment that has everything the cell needs, such as amino acids, vitamins and growth factors. With a few more adjustments it worked and that is when BR-1 was born.
Lygia concerned herself with improving the whole process before using the minute embryos that, as demanded by Brazilian Law, had been kept for more than three years in the freezers at the assisted reproduction clinics Fertility, in Sao Paulo, and Prof. Franco Junior in Ribeirão Preto. Even having mastered the technique the success rate is low: of the 250 initially thawed embryos most were no longer viable and could not be used for reproduction purposes. Only 35 developed to the 150 cell stage. Only the cells from one of these embryos managed to put down roots in the culture medium and begin to spread, like grass on fertile soil.
The product of two years work is now in dozens of ampoules stored in the freezer of USP’s Molecular Genetics Laboratory and also in the incubator and on the benches of the laboratory where the team is testing the cells to check whether they are truly pluripotent; so far it seems they are. Lygia’s team has shown that the morphology of the cells that reproduce on the glass plates is typical of stem cells. The group also genetically analyzed the cells and detected the activity of genes that are only expressed in pluripotent cells. The great test was to take off the brakes that maintain the cells in an undifferentiated stage. “The cells start to differentiate chaotically”, explains the researcher from USP, who saw neurons and muscle cells appear, which were recognized by their characteristics under the microscope and also by their specific antibodies. Important tests still have to be carried out to guarantee that the cells will have the functional versatility that is expected of them: the first will be to implant some of these cells in mice to see if they cause teratomata, structures in which the cells multiply and change into different types of tissue. “It forms a mass with skin, muscle, hair, teeth, the most varied of things”, says Lygia. It will also be necessary to carry out functional tests to know, for example, if the cells that look like neurons really produce potential action in response to an electrical stimulus, as they do in an organism.
Lygia and her helpers intend producing sufficient cells for all Brazilian groups that are interested in doing research with stem cells. Because of this it will be necessary to produce cells on a large scale, a task which neuroscientist Stevens Rehen has been working on in his laboratory at the Federal University of Rio de Janeiro (UFRJ).
The number of cells is unlikely to be the problem. In partnership with Leda Castilho and the PhD students Aline Marie Fernandes and Paulo André Marinho, all from UFRJ, Rehen has developed a technique that allows him to obtain 90 million cells in just 2 weeks, 70 times more than is possible in the traditional way of growing them, with a series of advantages in addition to efficiency. “We need to change the culture medium less often, which reduces the risk of contamination and makes the process three times cheaper”, said the researcher from Rio, who in order to multiply cells in a culture adapted an ingenious method already used to produce pharmaceutical products. Instead of cultivating them on plates which look like ice-making trays – if they were filled with water they would produce 6 ice cylinders some 3.5 centimeters in diameter – he transfers the cells to a bio-reactor. Despite its pompous name Rehen describes the bio-reactor as an enormous mayonnaise bottle with a magnetized plate and rod that shake the glass and fully control its contents. Furthermore, instead of leaving the cells to proliferate attached to the bottom of the plate, he adds microspheres to the mixture. After resting for some time the cells stick to the spheres and this is when the stirring begins. With the spheres in suspension this increases the area the cells have to occupy a lot. In addition, the stirring makes the environment more oxygenated and favorable to biological reactions.
The productivity expected by Rehen is more than enough to meet Brazilian demand, even with the waste there is likely to be at the beginning until the groups learn to keep the human embryonic cells in a culture medium with the help of Lygia and Rehen, who have offered to train anyone who is interested in working with them. But it is early days to be boasting of a victory. The neuroscientist from Rio is optimistic because his method worked with American cells. Even so it will be necessary to see if the BR-1 cells retain their pluripotent capacity after they have multiplied on a large scale.
 Photos Laboratory of Lygia da Veiga Pereira/ USPResearch instrument
Photos Laboratory of Lygia da Veiga Pereira/ USPResearch instrument
Lygia intends expanding the BR family. “It’s not clear how identical the strains are one to another”, she says; “We’ve noticed that each of them is more inclined to differentiate into one type of tissue, or another”. Furthermore, genetic peculiarities may be necessary for each line of research. Lygia herself, for example, is interested in understanding how the two X chromosomes, which together define that a human being is a woman, interact in a cell. This is important because in each of a woman’s cells there is a mechanism that makes one of the X chromosomes inactive – if not, the genes located in this chromosome would be duplicated relative to men. To understand how this works Lygia needs female cells, which is not the case with the BR-1, which was obtained from a male embryo. As they multiply cells change slightly. As a result old strains are more different from the original embryo than a more recently established cell. Comparing the properties of different strains is precisely the mission of the PhD thesis of Ana Maria Fraga, Lygia’s student who played a central role in establishing the Brazilian strain.
Despite the interest that news about stem cells arouses in the public, there is still likely to be a delay before therapies using these types of cell become a reality – not even the researchers hazard a forecast. Mayana Zatz never tires of repeating that the cells themselves are not a treatment: “We’re not going to inject embryonic stem cells into anyone”. So far experiments have shown that undifferentiated stem cells generate tumors when injected into animals. Researchers see them as a powerful research instrument, not a magic therapeutic solution. When they understand how embryonic stem cells give rise to all human tissue they will be able to interfere in the activity of the genes and the characteristics of the environment that will allow them to manipulate adult cells. “Embryonic cells are the only ones that have all the hardware”, explains Krieger, from InCor. “We need to understand the software and learn how to explore this software in other cells”. At this point, perhaps, embryonic stem cells may even stop being necessary.
In this same spirit the cardiologist is trying to discover what chemical stimuli transform embryonic stem cells into different components of the vascular system, such as heart muscle or blood vessels. The lines of research that promise the fastest result are those that use adult stem cells. In his laboratory Krieger has on-going clinical tests to check the capacity of cells from the bone marrow of the patient to correct ischemia, when the heart receives less blood than it needs. If it succeeds it will be possible to substitute, or at least complete the large number of by-pass operations, when a new blood vessel is implanted to substitute one that is blocked, and therefore restore the blood flow to the heart.

Laboratory of Lygia da Veiga Pereira/ USPAntibodies mark pluripotent cells (the two above), and transformed into neurons (nuclei in blue)Laboratory of Lygia da Veiga Pereira/ USP
Step by step
Krieger’s greatest enthusiasm is reserved for a project that does not involve stem cells, either embryonic or adult, and therefore shows that it may be possible to dispense with embryonic cells after their method of functioning has been revealed. The technique involves manipulation of skin cells or fibroblasts so that they produce a substance called vascular endothelial growth factor (VEGF). When these cells are injected into the vascular system they take a message to the cells of the circulatory system in the place where there is a deficiency: they produce more cells. Instead of implanting stem cells, which will change into cardiac or vascular tissue, the strategy is to stimulate local production. The work forms part of the PhD of Giovana Gonçalves, who defended her thesis this year. “It’s such a simple idea that people take a long time accepting it”, jokes the tutor. The results so far have been so good that Krieger’s group has already started tests on pigs, whose heart is much more like that of human beings than a rat’s heart. She is privileged to be at InCor, with access to cutting edge apparatus – the same used for human patients – for monitoring the hearts of pigs.
Adult stem cells are also the raw material of geneticists Maria Rita Passos-Bueno and Daniela Bueno, from USP, who removed muscles cells and the pulp from teeth for regenerating bone. Their objective, with promising results recently published on the website of the journal, Tissue Engineering, is to repair congenital cranial defects, such as hare-lips and cleft palates. “Embryonic stem cells are important, but we have to remember that the cells that naturally reconstitute the tissue when it is damaged are adult cells”, says Maria Rita.
Stevens Rehen, in turn, wants to understand how neurons differentiate themselves and how changes occur in the number of chromosomes. When – and if – he manages the formula for producing a specific type of neuron, the dopaminergics, he will have a good weapon against Parkinson’s disease, which his group is studying using mice as a model.
In the cell therapy symposium in Curitiba, where Lygia presented BR-1 to the community, Rehen showed the results of a second line of research carried out in collaboration with Ana Maria Martinez’s laboratory at UFRJ. The group caused lesions in the medulla of mice that were similar to those that can cause paralysis after an automobile accident. Compression kills both neuronal cells as well as those that form the myelin fat that coats neurons. Embryonic stem cells were implanted that were directed towards the neural path in which there is still flexibility to become a neuron or other type of nervous system cell, but never another type, like skin or muscle. The results were good: over a period of two months the mice recovered almost 60% of their mobility. If they had injected undifferentiated cells in this same time span they would have given rise to tumors, which is why it is necessary to direct them beforehand. “It’s like putting an 8 year old child in a university”, is the comparison the neuroscientist draws. “It’s better to let the child study and learn and send them to university later.”
Rehen has been using mice stem cells as well as human cells imported from the United States. “At this point in our research I’m not limited by a lack of Brazilian strain”, he says. The problem will arise when we get to the point of producing therapies based on these cells: the agreements would oblige Brazilian researchers to share any profits with the United States. “With BR-1 the patents will be 100% Brazilian.”
Mayana Zatz underlines that what is most lacking in Brazil is research structure. “Universities abroad have what they call core facilities, which are centers that produce the necessary products, like stem cells, transgenics or reagents”, she says. “Here, every laboratory has to know how to do everything on its own.” Even so her group has managed to gain international prominence with their research into stem cells for regenerating muscle and bone: along with Maria Rita Passos-Bueno, nine pieces of work have been published in international journals since last year. Despite being a defender of research with embryonic stem cells, Mayana has been concentrating more on adult stem cells where she hopes to get more immediate results. “I’m pleased that Lygia is willing to work with embryonic cells and help other researchers leapfrog stages. It was worth fighting to get this research released”, she adds. For Lygia even if everything goes well with BR-1, the strain is still the beginning of a long road. “We have the bricks and now we have to build the house from scratch.”
Republish