As they get older, the brains of capuchin monkeys, some of the most intelligent primates of the Americas, can present the same types of lesion that characterize Alzheimer’s disease. Researchers from the University of São Paulo School of Medicine (FM-USP) and the University of Brasília’s (UnB) Primatology Center identified, in two elderly animals — one 29 and the other 33 years of age — both beta-amyloid peptide plaque, formed of the buildup of these protein fragments around the neurons, and tau protein neurofibrillary tangles, which concentrate inside these cells and kill them. Neither the plaques nor the tangles were found in the brain of a nine-year-old young adult animal. The findings were published in the Scientific Reports journal in March, and according to the authors may pave the way for capuchin monkeys to be adopted as a natural model to study the evolution and treatment of Alzheimer’s disease, the most commonly occurring dementia in human beings (see Pesquisa FAPESP issue nº 329).
The idea of examining whether something similar to Alzheimer’s also occurred in capuchin monkeys arose years ago, when neurologist Ricardo Nitrini, of FM-USP, a scholar of dementia epidemiology in Brazil, and one of the research coordinators, met psychologist Maria Clotilde Tavares. A neuroscience and behavioral specialist, Tavares was already coordinating the UnB Primatology Center, home to the animals used in the study. “We awaited the natural death of these animals before evaluating the brain tissue,” reports Nitrini.
With sizes varying from 35 to 48 centimeters (tail not measured) and weighing up to 4.8 kilograms, capuchin monkeys (Sapajus libidinosus) are among the most common nonhuman primates in the Americas. In Brazil, they live in practically all biomes, from the most humid, such as Amazonia and the Atlantic Forest, to the driest, for example the Cerrado (wooded savanna), and the Caatinga (semiarid scrublands). They attract the interest of those investigating cognitive decline because, as well as being abundant, they present more sophisticated manual skills and behaviors than other monkeys, such as the fabrication and use of tools to hunt or extract chestnuts (see Pesquisa FAPESP issue nº 259). Two other characteristics can be added to these factors: the size of the brain in relation to the body — the so-called encephalization quotient, an indicator of intelligence — is superior to that of most other nonhuman primates, and they have a brain anatomically more similar to people, with folds and grooves, than other monkey species, particularly those native to the continent.
“These animals have a significant behavioral plasticity and adapt to different environments. I believe that, with this study, they will be more valued in the investigation of neurological illnesses,” says UnB’s Tavares. “Capuchin monkeys have a long life, and can live to 40 in captivity. This will allow study into how brain degeneration affects the skills of these animals over time,” says Tiago Falótico, researcher and current president of NGO Neotropical Primates Research Group (NeoPReGo). Falótico is examining the use of tools and cultural evolution of capuchin monkeys, and was not part of the study published in Scientific Reports, conducted with FAPESP funding, of the Brazilian National Council for Scientific and Technological Development (CNPq), Alzheimer’s Association, and National Health Institutes (NIH) of the United States.
Until recently, neurologists and neuroscientists conjectured that Alzheimer’s disease was an exclusively human illness, because there was no knowledge of other animals presenting the two typical types of lesions. This began to change in 2008, when Lary Walker and his team at Emory University in the US reported, in an article published in The Journal of Comparative Neurology, to have found beta-amyloid peptide plaques and tau protein tangles in the brain of a 41-year-old female chimpanzee.
Roberta Diehl Rodriguez / USPHigh encephalization quotient: capuchin monkeys’ brains (real image) are proportionally large in relation to their bodiesRoberta Diehl Rodriguez / USP
Originating in Africa, these simians are, in evolutionary terms, the closest living primates to human beings, sharing a common ancestry from between 7 and 5 million years ago, with almost 99% identical genes. For this reason, from a biological standpoint, chimpanzees appeared to be a good model to investigate the evolution and treatment of illnesses afflicting Homo sapiens, including Alzheimer’s disease. However, maintaining a chimpanzee in the lab is not inexpensive — according to some estimates, it can cost over US$20,000 per year — and these animals are at risk of extinction (there are fewer than 300,000 in nature). Furthermore, little more than a decade ago, animal research directives became stricter (see report). US and European healthcare authorities started to recommend that chimpanzees only be used to investigate human illnesses when there was no other model available, or when it was not possible for ethical reasons to carry out these tests on people.
Given the impossibility of conducting certain experiments on human beings, researchers have advanced their understanding of the phenomena behind Alzheimer’s and the quest for more effective potential treatments based on initial studies with models that are not always ideal. The experiments generally begin with cell culture in the laboratory, enabling investigation of the gene activation pattern and modifications in the biochemical pathways occurring during illness, and progress to tests on rodents, almost always mice, which can indicate how the illness affects certain cognitive aspects. The variety of models applied to understand how the illness takes hold and evolves is comprehensive, ranging from worms to fruit flies; from fish to other primates, such as lemurs and some monkey species. None of these, however, faithfully and completely reproduce what happens in the human brain.
Invertebrates, for example, are advantageous in certain situations, because they share certain genes relevant to Alzheimer’s disease and reproduce more quickly than mammals. However, although they can be useful for learning about the biochemical pathways affected by the disease, they are not ideal for studying treatments because the effects that the compounds produce in them are not always observed in animals with a more complex nervous system.
By far the most used in research, rodents allow observation of certain behavioral effects of the disease, such as the loss of spatial memory, and certain benefits of potential therapies. They do not enable full reproduction of the illness, however. “Mice and rats do not spontaneously develop beta-amyloid peptide plaques, nor tau protein tangles, although the first type of lesion occurs naturally in elderly specimens of a rodent known as the degus, found in Chile,” says biochemist Sergio Teixeira Ferreira, of the Federal University of Rio de Janeiro (UFRJ), who investigates the causes of Alzheimer’s.
For at least two decades, researchers have tried to navigate this limitation by artificially manipulating the animals. One of the strategies is genetically altering rodents to make them produce beta-amyloid plaques or tau tangles. Another, developed by the UFRJ group, involves the injection of beta-amyloid peptide fragments (oligomers) directly into the mouse’s brain. “This causes alterations very similar to those brought about by Alzheimer’s disease, including memory loss,” explains Ferreira.
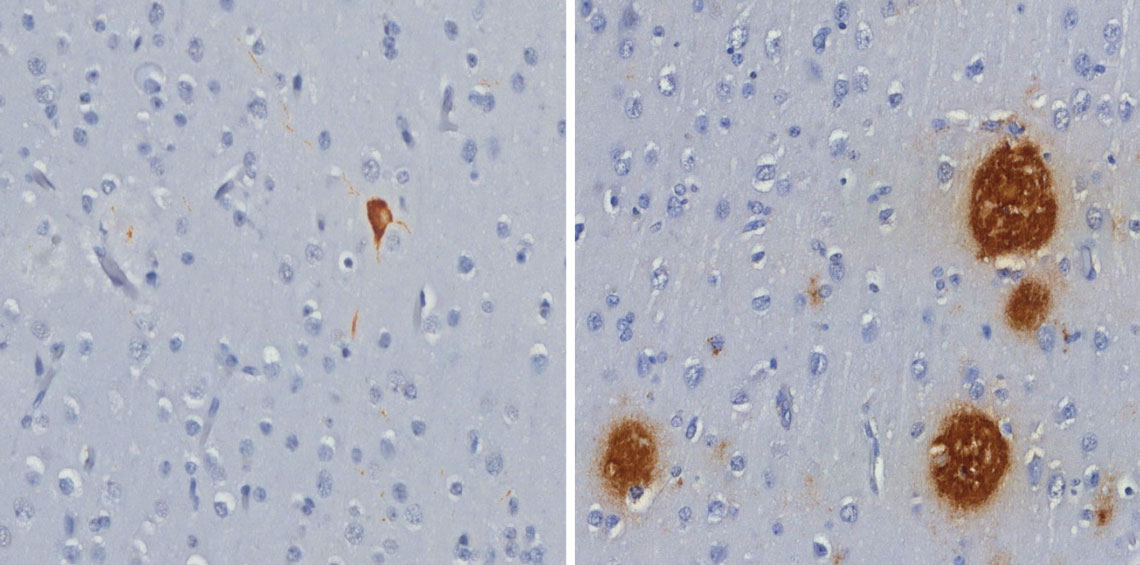
Roberta Diehl Rodriguez / USPTau protein tangle (left) and beta-amyloid peptide plaques (right), both in brown, found in the brains of capuchin monkeysRoberta Diehl Rodriguez / USP
Even so, what happens with the rodents can be very different from what happens in the brains of humans. Mice, for example, only share 85% of their genes with human beings, and have a much simpler brain anatomically. In rodents, the organ has a higher proportion of neurons than other cells — in the human brain, the proportion is approximately one to one. Researchers estimate that this difference can make the disease develop in animals differently from the way it evolves in humans.
“Despite the success, the two-dimensional models of cells and animal models can only capture a fraction of the mechanisms of Alzheimer’s disease, because they are incapable of recapitulating the specific structure, function, and cell diversity of the human brain,” wrote bioengineer Donghui Zhu and colleagues of Stony Brook University, in the US, in a 2022 review article published in the journal Bioengineering and Translational Medicine.
One way of closing the gap between the human illness and animal models is to use phylogenetically closer species that spontaneously develop the typical pathology of Alzheimer’s. “Working with natural models allows observation of a more realistic condition, more similar to what happens in humans,” says Analía Arévalo, a neuroscientist specializing in language, and a researcher at the Experimental Surgery Research Laboratory at FM-USP, who did not participate in the Scientific Reports study.
Even among primates, with more complex cerebral architecture than rodents, and evolutionarily closer to humans, the perfect model has yet to be found. Beta-amyloid peptide plaques have been registered in rhesus monkeys (Macaca mulatta), cynomulgus macaques (Macaca fascicularis), white-tufted marmosets (Callithrix jacchus), and gray mouse lemurs (Microcebus murinus), but they do not always occur simultaneously with the tau protein tangles, though the animals may present cognitive deficiency. Another difference is observed in the brain regions where these plaques and tangles form. In humans, they occur more frequently in the hippocampus, the area associated to memory acquisition and consolidation, while in marmosets and rhesus monkeys, they are more common in areas associated to the emotions (limbic system) or hearing (temporal cortex).
In these two aspects, capuchin monkeys may provide a closer model to the human disease: they presented beta amyloid plaques and tau protein tangles, and these lesions affected both the cortex and the hippocampus, with researchers also identifying signs of neuroinflammation, as also occurs in humans. “No other New-World primate used in the studies is as intelligent as the capuchin monkey, which makes its own tools and can remain in a biped position for quite some time,” says neuroscientist Roberta Diehl Rodriguez, lead author of the Scientific Reports study. “Moreover, the New-World primate presents more similar neuropathological alterations to those of human beings,” she states.
For capuchin monkeys to effectively become a model for Alzheimer’s disease, however, researchers will need to confirm the occurrence of lesions in a greater number of animals, and characterize how they affect behavior. “This will be the most interesting aspect to compare with the disease profile in humans,” says Arévalo.
Even if it works, there is a limitation. The disease in these animals may take decades to progress. To overcome this issue, neuroscientist Fernanda De Felice’s group at UFRJ is seeking an artificial Alzheimer’s model in young primates. Ten years ago, she and colleagues from Queen’s University in Canada successfully reproduced in cynomulgus macaques — currently endangered — the damage that Alzheimer’s causes in humans through injection of beta-amyloid oligomers into the animals’ brains, which accumulate in the frontal cortex, the hippocampus, and other areas associated to memory and cognitive aspects (see Pesquisa FAPESP issue nº 225). More recently, the group induced lesions in young rhesus monkeys between 3 and 5 years of age, to avoid waiting for the natural evolution of Alzheimer’s. “If we can reproduce the disease in young animals,” says Ferreira, of UFRJ, and husband and collaborator of De Felice, “it would make the work much easier.”
Project
Characterization of tau astrogliopathy in aging and neurodegenerative diseases (nº 16/24326-0); Grant Mechanism Postdoctoral Fellowship; Supervisor Ricardo Nitrini (USP); Beneficiary Roberta Diehl Rodriguez; Investment R$301,733.62.
Scientific articles
RODRIGUEZ, R. D. et al. Bearded capuchin monkeys as a model for Alzheimer’s disease. Scientific Reports. mar. 15, 2024.
ROSEN, R. F. et al. Tauopathy with paired helical filaments in an aged chimpanzee. The Journal of Comparative Neurology. may 14, 2008.
SREENIVASAMURTHY, S. et al. Current progress of cerebral organoids for modeling Alzheimer’s disease origins and mechanisms. Bioengineering and Translational Medicine. jul 29, 2022.
