Glaucius Oliva’s passion for taming proteins for drug development began during his undergraduate studies, as an engineering challenge: to discover how to bind dozens of plastic pieces to form a myoglobin molecule. It took months of work to calculate the exact position and orientation of each atom and to place all of the pieces, puncturing and fitting them onto wires precisely cut to size. It was then that the electrical engineering student knew what he wanted to do.
He just didn’t know that this scientific interest would also require that he dedicate himself from the beginning of his career to bring together specialists from different areas to work together and convince funding agencies and research institutions to support them. Thus, Glaucius Oliva became a top administrator, both of large interdisciplinary projects, such as the Brazilian National Council for Scientific and Technological Development (CNPq), which he headed between 2011 and 2015. As a Professor at the Institute of Physics at the São Carlos campus of the University of São Paulo (USP), he currently directs the Center for Research and Innovation in Biodiversity and Drug Discovery (CIBFar), one of the Research, Innovation, and Dissemination Centers (RIDC) funded by FAPESP.
Oliva gave this interview in his office, where the molecular model has been on display for almost 40 years, in an institution that was almost empty on Christmas Eve. His laboratory was in full operation.
Specialty
Crystallography, structure of proteins
Institution
São Carlos Institute of Physics at the University of São Paulo (USP)
Education
Bachelor’s degree in electrical engineering (1981) and master’s in physics from USP (1983), doctorate in crystallography of proteins from the University of London (1988)
Scientific production
163 scientific articles
Your work has put you on top both as a manager and as a researcher. What is your greatest strength?
These two things are intertwined in my story. I have always been involved in research. Even while holding positions in administration, I was in the laboratory, because I think it’s dreadful when a researcher becomes a manager and forgets his life passion. In general, disconnecting from one’s core work distances you from difficulties, problems, and challenges. Management in science and technology means you have to make daily choices about how to use resources to reach objectives.
Are they also intermingled in the management of a research center?
Yes. I have always managed large projects, beginning with the first round of thematic projects funded by FAPESP soon after I finished my doctorate in 1988 and returned from England. We were able to build the laboratory team because of this. Later, other projects came along because our area is very interdisciplinary. We are at the Institute of Physics, but we need biology, computer science, chemistry. At the beginning, collaboration was essential among the groups that produced the proteins in order for us to study them.
Does the RIDC have a large management team?
We are a team of 22 lead researchers, as well as students, and we have administrative support. One of the challenges of managing a project such as this is integrating the researchers. As they are all talented and independent, it is necessary to convince them that collaborating with each other is worth the effort. If each continues doing only his or her tasks, we will have a strong list of publications for the report. But the focus of CIBFar is drugs, and Brazil does not have a history of innovation in this area. We need the biologist, the structural biologist—who studies molecular structure—and the chemist. And someone who oversees everything, the manager. I no longer enter the laboratory to work with test tubes, but I go in every day.
There was no place where you could study a promising substance in a collaborative way
From research to production, what is missing?
The national pharmaceutical industry is very well equipped with respect to formulation and production. With legislation having regulated generic brands, it was necessary to learn how to make qualified drugs. Now, the challenge is innovation. Imagine that the drug must find a receptor to fit into in such a way that produces a certain effect, which is to activate or deactivate a certain protein. Finding a molecule is not easy as we have thousands of proteins in our bodies, with thousands of different structures. Side effects generally occur when the drug molecules stick to things they find along the way. Domesticating a molecule that runs throughout the organism until it reaches its target is an art that still requires trial and error, with synthesis of different molecules.
And each illness works differently?
With microorganisms, it is necessary to identify the protein that is essential for its survival, so that we have a validated target. Many people study the microorganisms that cause diseases, specifically how they produce symptoms, attach themselves to the cell they want to invade, and inject into it genetic material or proteins. I always worked with the Chagas disease, schistosomiasis, leishmaniasis, but other colleagues were taking care of this while I was at CNPq, between 2011 and 2015. When I returned, it was the time when the Zika epidemic was happening. So we started a project about arboviruses and today we are working with Zika, yellow fever, and chikungunya. Some decades ago, the process was very experimental and happened through random findings. It changed when we understood better the biology of the diseases and began to study the structure of the proteins that interact with them.
A large group is needed to handle the chain of knowledge. Do you need more people?
What’s missing is linking everyone. We have a researcher who understands pharmacokinetics in mice, another that knows toxicology, others that study the fundamental biology of diseases, who work with the structure of proteins or chemical synthesis. But we need an orchestra. If everyone concerns themselves only with their own sound, it will never become a symphony. Brazilian science is still extremely disciplinary. At the universities, departments are segregated. Undergraduate courses are isolated from each other—you can’t find a student who understands the physics of using an X-ray machine and biophysical techniques, such as fluorescence, and, at the same time, knows what a bacteria is and the molecular biology of cloning a gene.

Personal archive
José Fernando Perez, Francisco Landi (then-directors of FAPESP), Flávio Fava de Moraes (then-chancellor of USP) and Oliva at the launch of the experiment onboard the Columbia in 1997Personal archiveIs CIBFar an evolution of the past?
We presented our first proposal in 1998 during the RIDC’s first invitation. The program was created because we said to José Fernando Perez, the then scientific director of FAPESP, that modern science is multi-disciplinary. I saw this because, in 1996, we were working with the Chagas disease, a subject that allowed us to build a light source in the LNLS [Synchrotron Light Laboratory] to analyze the structure of proteins. In order to do this, crystals are required, where atoms are organized. But, in one crystal measuring one tenth of a millimeter, there are 1013 protein molecules: 1 followed by 13 zeros—various trillions of molecules that need to be organized—it’s a fair bit of work. Then, while at a conference, I met an American biochemist named Lawrence DeLucas, from the University of Alabama in Birmingham, who was an astronaut working for NASA [North American Space Agency] on a project involving protein crystallization at microgravity—at these conditions, crystals form more easily. He invited me to do an experiment on the International Space Station, which became the first Brazilian project to be carried out in space, launched on the Columbia Space Shuttle in 1997. Upon my return, I visited the laboratory in Birmingham, a center of the National Science Foundation [NSF]. They had research centers with this multidisciplinary approach and my colleague was in charge of one of them, with biologists, chemists, as well as people from IT, crystallography, and drugs, working in collaboration and in conjunction with companies. I suggested that we have something similar and that professor Perez should go to the United States and visit the NSF centers. When he returned, it was a go. Our challenge, in the project we presented, was to create in Brazil a critical mass of biological materials. No one in the country was producing pure proteins. We had 15 biologists and molecular biologists, and two chemists. Today, the challenge with drugs is chemistry and, in the current center, we have flipped the ratio: we have 15 chemists and two or three structural biologists.
And “biodiversity,” in the name CIBFar, where does it come in?
It comes in as a source of inspiration for active molecules. The drug cannot be too polar because, if it were, it would not cross any membrane; it cannot be too hydrophobic, or it would not be soluble; it cannot be too labile or it will break; and, at the same time, it cannot be too large, or it will stick to other things. A plant does not run, does not yell; it is still. Its only defense against fungi, insects, phytopathogenic bacteria is chemistry. The biosynthetic paths that these molecules make have been selected over the course of billions of years of evolution, and they were domesticated to interact with other living organisms. They can be used to make modifications in order to optimize their behavior and simplify the chemistry. Later, we will be able test in vitro properties without using animals. Before, there was no place where we could study a promising substance in such an integrated way. This is what CIBFar does.
Are there noteworthy results?
Recently, in a project managed by my colleague Rafael Guido, we found a set of molecules to fight malaria. The patent is still pending. They are extremely powerful molecules, with promising pharmacokinetic properties, that have killed parasites in vitro and in vivo in experiments with mice. This attracted the attention of GlaxoSmithKline, an international pharmaceutical company that contacted us through FAPESP. The molecules were transferred to the Glaxo development center in Tres Cantos, Spain, through an agreement, and right now are being put through tests that are not carried out in Brazil. Another example of a discovery by members of the RIDC at the Federal University of São Carlos is a natural molecule called 10-gingerol, extracted from ginger, which has proven to be effective in inhibiting metastasis of breast cancer. We have new molecules to fight yellow fever, for example, with a very significant potency that has yet to be described in literature. They are promising results. In the RIDC’s first six years, we have brought people from different areas to work together. Now the structure is built and it’s time to deliver results.
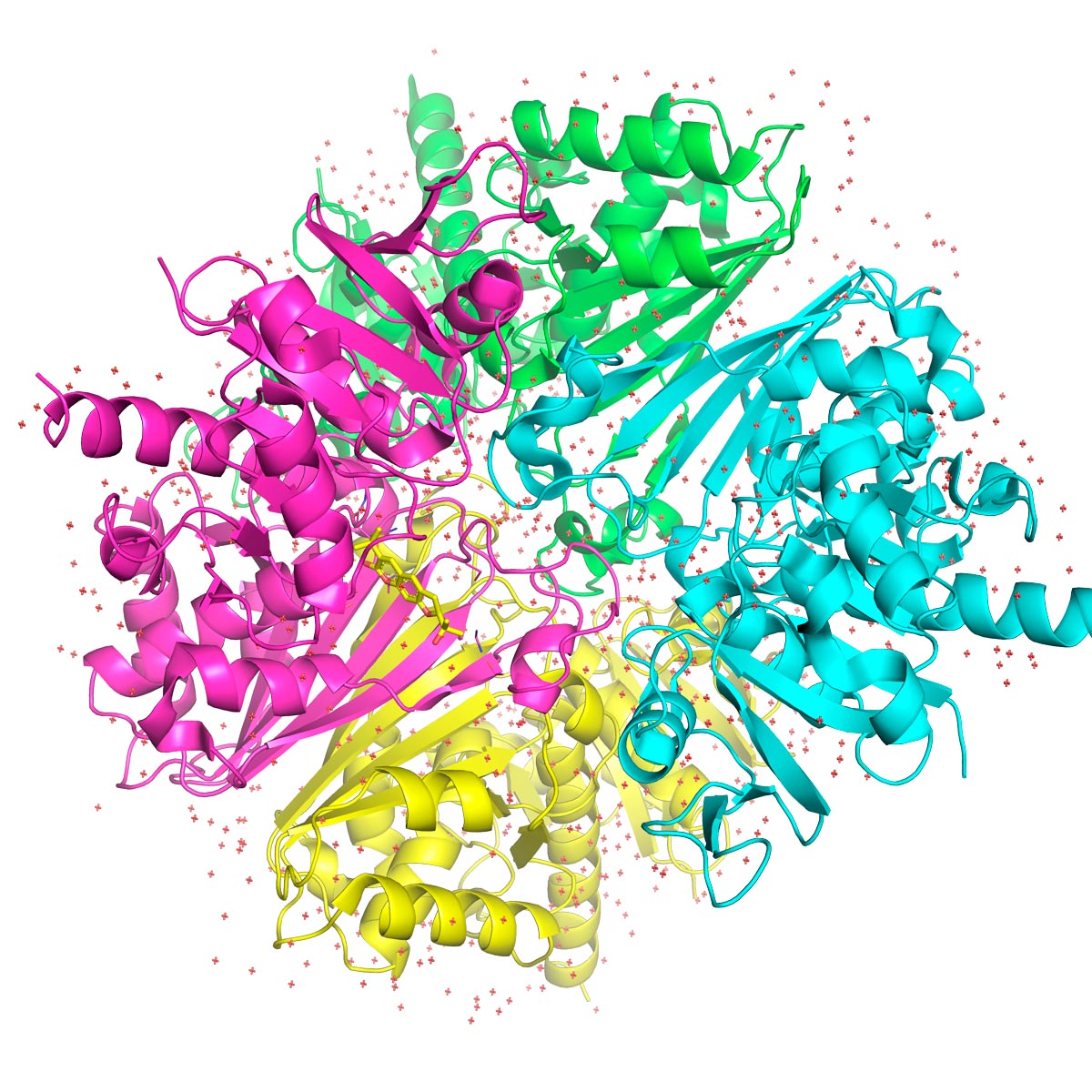
CIBFar
Complex models of molecules are generated today by computer; here, a GAPDH protein of Trypanosoma cruzi CIBFarYou are using Synchrotron Light to illuminate the structure of proteins. Now, a new generation of particle accelerators has arrived with the Sirius. What will change?
A lot will change. During my doctorate in 1987, it took me a weekend to collect a set of X-ray diffraction data for a protein and six months to process and analyze the 600 photo boards. Today, we send tanks of liquid nitrogen with protein crystals to the Diamond accelerator in England. Each data collection takes close to 30 seconds. The Sirius will facilitate an increase in the X-ray diffraction resolution, significantly reduce the size of crystals, and generate analyses much faster than the alternatives we have here and around the world.
You graduated from engineering. Did you ever think about another professional path?
It was by chance. I chose electrical engineering, but at the end of the first year, a professor who taught me physics invited me to help build a vacuum system for some experiments during school break. Professor Sérgio Mascarenhas was the head of the biophysics lab and was always inspiring and motivating. I attended his introductory class of the history of physics and I loved it. In the second term of 1978, he had returned from England and showed me a box that I didn’t know what to do with. It was a set of pieces representing atoms of a myoglobin molecule, but he had lost the instructions and asked me if I could assemble the model. It took me six months and I discovered that that was what I wanted to do.
What did you do to become a physicist?
In the second year of engineering, I decided to do another entrance exam. At that time, it was possible to do two degrees at the same time. Both were daytime programs, but I got used to it. I was already very immersed in the laboratory of chemist Yvonne Mascarenhas and physicist Eduardo Castellano, an Argentinean who escaped his country’s persecution, who was doing X-ray diffraction in small molecules. No one in the country was doing the structure of proteins. When I finished engineering, a lecturing physics professor—Aldo Craievich—transferred to the Brazilian Center for Physics Research in Rio de Janeiro. At that time, in 1981, there was no public competition for professorships and the departmental council decided to contract four professors to replace Craievich as teaching support staff: Vanderlei Bagnato, who managed another RIDC and the director of the Institute of Physics; José Nelson Onuchic, today a member of the National Academy of Sciences in the United States; Carlos Antonio Ruggiero; and myself. Carlos Antonio and I did not yet have our degrees. The others had graduated the prior year and were immediately contracted. For this reason, my contract date as a professor with USP is December 18, 1981, the day I got my degree.
We changed the institution’s undergraduate program to take students out of the classroom and get them working
Did you complete your physics degree?
In the beginning of 1982, I went to register and they told me that I couldn’t be a student if I was a professor in the same institute. So I registered for a master’s in the structure of small molecules. It was the preparation for studying the structure of proteins, which is what I had always wanted to do, and I started my doctorate at the University of London in England, with Tom Blundell, an international leader in the structure of proteins. Over four years, I studied three proteins: a small peptide, another medium-sized one, and a very large protein associated with neurodegenerative diseases, such as Alzheimer’s and amyloidosis. It was this disease where it took six months to do the data collection and processing. Then, to sort out the structure it took two more years, which landed me an article in Nature in 1994.
Upon returning to Brazil, what did you do next?
There was no infrastructure that combined protein preparation laboratories, crystallization labs, and X-ray labs. When I arrived, director Oscar Hipólito said that he had a lab for me. It was a room with a sink covered in white tiles. Normally, a physics lab would not have a sink, but he thought that water was needed to work with the structure of proteins. Water isn’t needed to use a laser; it actually creates problems. It was challenging as we began to collect proteins from people’s laboratories. It was all very handmade and there was not enough material to work with. We had to be creative.
What happened during the election for chancellor when you won the popular vote, but were not elected?
In 2006, I was elected as director of the institute. Speaking with chancellor Suely Vilella [2005–2009], I saw that she was continuously solving day-to-day problems—money, RFPs, student cafeterias—but we had fundamental problems to think about: where USP was going, how we could rethink the undergraduate program… There was no time to think about USP in the medium to long term. Then, I discovered in the USP statutes, from 1988, that there was a planned commission. I spoke with the chancellor and, at the end of 2007, she called the commission over which I presided. We booked meetings that lasted an entire day each month, more or less. We would go to different sites—Piracicaba, São Carlos, São Paulo—and we had the day to discuss key issues. We brought people from abroad to speak about departmental structure and about the undergraduate program, if the university needed to be research-focused or teaching-focused, if every site had to have the same model. A book even resulted from this, called USP 2034 – Planejando o futuro (USP 2034 – Planning the future). In that year, when the election for chancellor arrived, we realized that this needed to happen for a future-focused university, and so the idea was born to have a candidate for chancellor. In the first months of 2009, we turned this into a management project. In June, I became a candidate; the university community liked having a project that was developed by so many people. I was the one who received the most votes in the first and second rounds. But the then-governor José Serra chose Grandino Rodas.
So then came CNPq.
I was the director of the Institute of Physics, and I still had six months left in my term, but it was uncomfortable. The director has a seat on the University Council… It would have been awful for me and for Rodas, as chancellor. In 2010, a physics colleague from Rio, Carlos Aragão, was named president of CNPq and invited me to be the director. At the end of that year, Aragão left, coincidentally with the change in government. Dilma Rousseff became president, and Aloizio Mercadante became minister, and he ended up naming me president of the institution.
After being considered in the first call by the National Institutes of Science and Technology (INCTs), you saw the program from the other side. How did you see it?
We had 120 funded INCTs since 2008, and in the call for proposals, an evaluation was planned for. In 2010, and later in 2013, we organized three-day events in which the managers of all of the INCTs, sometimes one or two more individuals, would go to Brasília. Each one of them had a display where they showed what they did in a visual way. We brought people from the city, from government, from congress to see what was being produced. An international committee of about 20 people came. We organized a symposium divided into topic areas, with each INCT having an hour to present and state their case, and everyone else from the same area attended. It was fantastic as the INCTs got to know each other and people from abroad saw what they were doing. The cost-benefit was very positive.
Has this allowed you to think in a broader sense?
Yes, but one of the things I accomplished that I am most pleased about were the themed callouts. I wanted to change the CNPq from being a development agency of the Ministry of Science and Technology to a development agency of the Brazilian government. The directors who worked with me had to go to the ministries to find problems to be solved. While they invested a lot of money, we contributed a small amount and launched the callouts with specific themes. For example, meaningful technologies for the “Minha casa, minha vida” (My home, my life) program, technologies that were appropriate for small rural properties, health—such as the dengue problem. The ministries gave out money. I did callouts with Petrobras for oil and gas projects, and with Vale. The companies invested.
The role was to organize?
Not only that; the CNPq has the tools and can grant scholarships, for example. The scientific community is ready to take care of the country’s needs. We are thought of as an alienated bunch living within the walls of the universities, concerned only with ourselves and the papers we have to publish. It’s not like that. When called upon, scientists contribute to society. If they’re not called, they focus on what the funding agencies require.
How did the Science without Borders program come about?
When I arrived at CNPq, there were only 500 foreign scholarships per year available. How can we live with such a rustic kind of science? In that same year, Obama came to Brazil and mentioned to Dilma that in the US they had launched the 100,000 Initiative, which motivated American universities to send 100,000 students to see the world. This is what is called the diplomacy of science: when you send a student abroad and he lives in a community and builds a network of highly qualified people, which later generates benefits far beyond education itself. President Dilma ended up doing the same and we proposed that postgraduate scholarships would be predominant, but demand was extremely low. Our students were in an unrealistic comfort zone. If they got into a doctorate program they were sure to get the degree. They had scholarships and jobs available in Brazil. There was no motivation to leave the country.
People from the undergraduate program thought it was fantastic.
Yes. The big villain for the structure of Brazilian science is the undergraduate program, with this compartmentalized system where the various disciplines don’t communicate with each other, nor do their courses. Undergraduate courses are all about lectures. It is quite common to have an engineering student with 35 or 40 credits per term.
Is there no time to be creative?
[The student] sits, watching the professor who writes on the board. When a professor claims to have “updated” the class, what they mean is they have added PowerPoint slides. I said that we needed to update the Brazilian undergraduate programs. No foreign programs have more than 15 or 16 hours of class per week. Students go to the library because they have to turn in reports, write oral exams, and there is a lot of pressure. Here, the currency for contracting university professors is class time. The more disciplines offered, the more professors that can be contracted. The end result is an inflated teaching schedule for professors who don’t have time for creativity or innovation. The student is passive throughout the undergraduate program and is expected, on the first day after graduation, to get a job and be innovative in his new company. Brazilian companies are not innovative because students have not been trained to be innovators. If we were to send them abroad, they could begin to revolutionize these courses by incorporating what they learn in other countries. It was an expensive program, that’s for sure. But we sent 100,000 students abroad.
What did you bring back to the university from this experience?
I worked very hard, with other colleagues, to change the physics undergraduate program. We have three undergraduate programs in the institution: traditional physics, computational physics, and biomolecular physics. They were considerably independent of each other, so we brought them together in the first year, reduced the required class load to 50% of the standard load. The other 50% students could build with elective activities from any one of these programs, but also from other campus faculties. The final scientific research paper counts as credit toward the undergrad degree, and our objective is to take the student out of the classroom and get him working. We are now in our second year of this new undergraduate program. I am now in a new phase of my life where I have submitted my request for retirement. Nothing will change: I will continue as a senior professor with this laboratory and research project, with my postgraduate students and teaching undergrad classes. The only thing I can’t do is participate in council and fellowship meetings.
Nor be chancellor.
This way people will stop calling me every time there is an election for chancellor, asking me if I’m going to run. Honestly, I want to spend more time with my two little granddaughters, who are one and five years old.


