The initial results of small tests carried out in countries such as the UK and Spain indicate that so-called heterologous COVID-19 vaccination regimens are safe and effective. In certain cases, there is evidence that mixing vaccines produces a better immune response than two doses of the same vaccine. With this alternative immunization regimen, the first dose uses a different vaccine to the second. The idea of combining different vaccines has been floated repeatedly due to the lack of doses needed to complete the traditional vaccination schedule in some parts of the world. With the exception of Janssen’s vaccine, which only needs one dose to provide full protection, all the other approved vaccines require two doses.
Another motivating factor for the approach is the incidence of some very rare but serious side effects, such as thrombosis after a first dose of the Oxford/AstraZeneca vaccine, especially among women. Individuals at higher risk of side effects who have already received a first dose of the AstraZeneca vaccine could be advised to take a different vaccine for their second dose. In several countries, including the UK, Spain, Sweden, France, Italy, and Canada, people given a first dose of AstraZeneca are allowed to receive a different vaccine for their second dose, usually the Pfizer/BioNTech vaccine, which uses messenger RNA (mRNA) technology. On July 26, the Brazilian Ministry of Health officially recommended use of the Pfizer vaccine or CoronaVac as the second dose for pregnant and postpartum women who had initially been given an AstraZeneca vaccine. The city of Rio de Janeiro adopted this strategy at the end of June.
Data from a University of Oxford study with 830 volunteers aged over 50 indicate that there are no problems with combining vaccines that use different technologies. The study, the results of which were published online as a preprint (no peer review) in June, analyzed the use of two combinations. The first involved a first dose of the Pfizer gene vaccine, which uses mRNA technology to produce SARS-CoV-2 antigens, and a second dose of the AstraZeneca vaccine, which uses a monkey adenovirus (which is harmless for humans) to introduce the body to the novel coronavirus spike protein and thus stimulate an immune response. The second approach used the same two vaccines but reversed the order.
Tomaz Silva / Agência Brasil
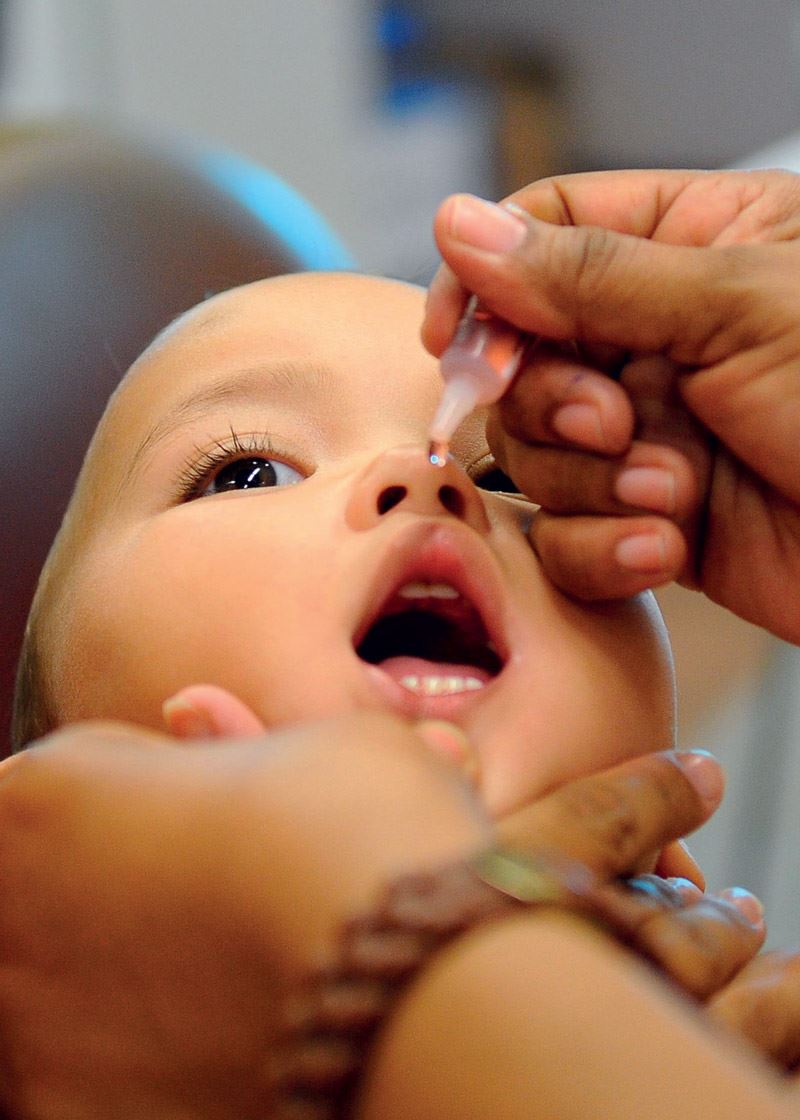
Different vaccines are combined to immunize people against polio, one of which is injected…Tomaz Silva / Agência BrasilBoth combinations led to increased SARS-CoV-2 IgG antibody concentrations in the blood and greater T cell production. “The results show that when given at a four-week interval, both mixed schedules induce an immune response that is above the threshold set by the standard schedule of the Oxford/AstraZeneca vaccine,” summarized Matthew Snape, a virologist at the University of Oxford and lead researcher of the study, in a press statement issued at the end of June. Snape’s group is yet to publish the results of trials with a longer interval of 8 to 12 weeks between doses. This regimen is the most common strategy adopted in countries administering the Oxford/AstraZeneca vaccine.
It is also the case in Brazil, where the British vaccine is manufactured at the Oswaldo Cruz Foundation (FIOCRUZ) in Rio de Janeiro. “The results of this study are very positive, showing that it is possible to reduce the interval between doses of different vaccines while maintaining a good immune response,” says immunologist Daniel Youssef Bargieri, head of the Vaccine Research Center at the University of São Paulo (USP). According to him, vaccines that use different technologies can complement one another because they act differently in the body. “Vaccinology is not an exact science. It unites knowledge, research, and empiricism,” says Bargieri. “From an immunological point of view, it makes perfect sense to consider using different vaccines. This is nothing new.”
In May, before the British study, a group from the Carlos III Health Institute in Madrid, Spain, announced preliminary and promising data from its CombiVacS clinical trial. The study analyzed the immune response and safety of heterologous vaccination in a group of 663 people aged between 18 and 60. Two-thirds of the volunteers received a first dose of the AstraZeneca vaccine and then a Pfizer dose at least eight weeks later. The other third—the control group—were given only the first dose of the British vaccine during the study period. Two weeks after receiving the Pfizer dose, the individuals taking the combination of the two vaccines had 150 times more COVID-19 antibodies than the control group. The heterologous vaccination procedure resulted in only mild and moderate reactions, which all disappeared two to three days after administration of the second dose. The Spanish study was published on a preprint repository at the end of May.
Tomaz Silva / Agência Brasil
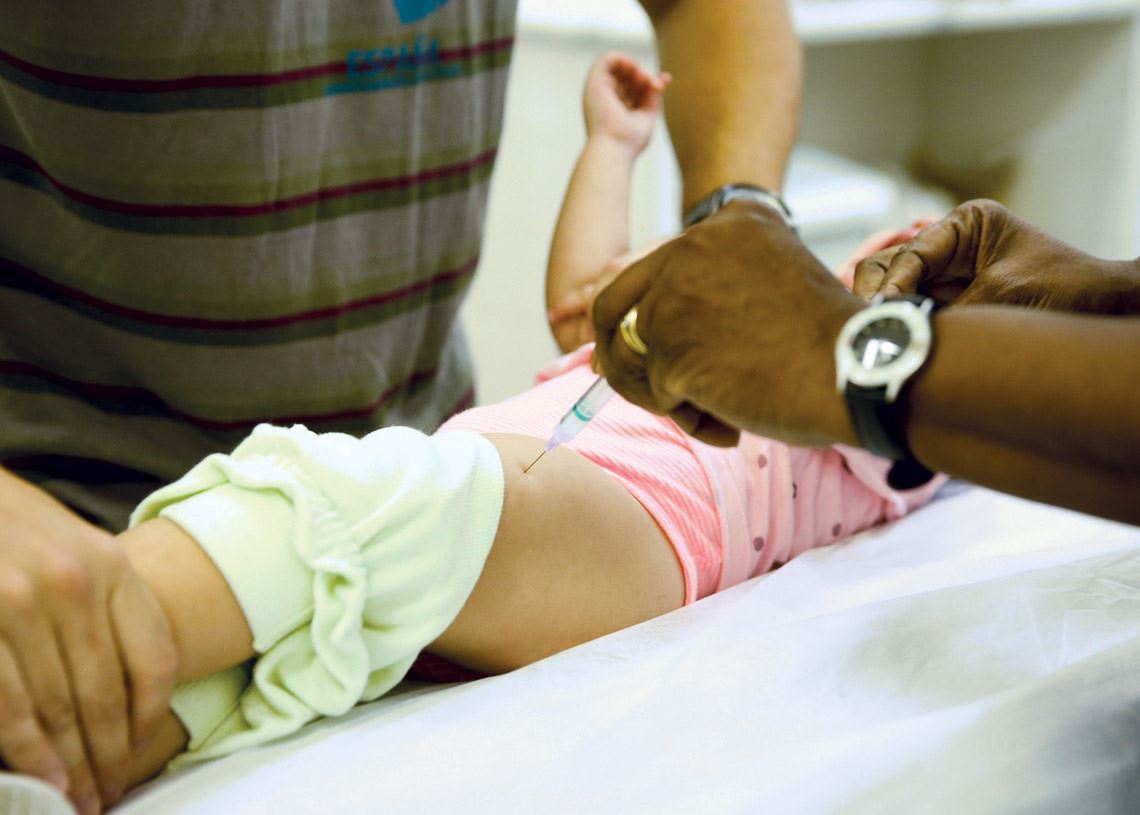
…and the other administered orallyTomaz Silva / Agência BrasilWith the novel coronavirus continuing to circulate among humans, which could lead to booster vaccines being needed every one or two years, alternating the type of vaccine used could contribute to the success of future vaccination campaigns. It would be beneficial both from a logistical point of view—the vaccines most available can be applied—and in relation to the immunological control of COVID-19. Long-term use of the same vaccine could result in a loss of efficiency over time, especially when the virus is constantly evolving and new strains of SARS-CoV-2 are continuously emerging.
Immunization against polio is always cited as a successful example of combining the use of vaccines that use different technologies. One of the vaccines, Salk, is administered by injection, and the second, Sabin, is given orally. The Salk vaccine uses an inactivated form of the virus. The Sabin drops, however, contain a live but attenuated version. Currently, children are initially given Sabin, with Salk used later in life as a booster.
“It makes perfect sense to mix vaccines because the strategy induces a broader immune response against the coronavirus,” says immunologist Alessandro Farias, from the University of Campinas (UNICAMP). Alongside his daily work, the researcher led a task force at Campinas that developed COVID-19 tests in the early months of the pandemic in 2020. “Over time, the immune system could learn to respond to the adenovirus in the AstraZeneca vaccine before the vector is able to fulfill its role of carrying the SARS-CoV-2 spike into cells. If that happens, the vaccine will lose effectiveness,” explains Farias. The researcher believes that alternating vaccines will also be important when it comes to distribution over large geographic regions, such as the interior of Brazil. “But at the moment, we are facing a different problem: we still don’t have enough vaccines for everyone,” he points out.
Although British studies show that combining different vaccines produces robust immune responses regardless of the order, some differences were observed. The AstraZeneca-Pfizer combination, in this order, induced more antibodies and T cells than a Pfizer-AstraZeneca approach. Both combinations, however, generated more antibodies than the standard two-dose application of AstraZeneca. The regimen that produced the most robust immune response was two doses of the Pfizer vaccine.
According to Andrew Ustianowski, clinical leader of the COVID-19 vaccine research program at the UK’s National Institute for Health Research, it is important to advance our knowledge of how the new vaccines function against the coronavirus. “The Oxford-AstraZeneca two-dose schedule is highly effective and has helped to save many lives. The fact we now know it works well when combined with the Pfizer vaccine provides greater flexibility to vaccinate more people globally,” said Ustianowski in a press release when the first results of the vaccine mixing study were announced in June.

Steffen Seibert / German Government
Angela Merkel’s vaccination card shows that the German chancellor’s first dose was AstraZeneca’s COVID-19 vaccine, but, according to the media, her second dose was with the Moderna vaccineSteffen Seibert / German GovernmentIn Germany, two preprints published on medRxiv in June described studies that reached similar results to those obtained in the UK and Spain. In one study by a team from Charité Universitätsmedizin in Berlin, the safety and efficacy of a combined regimen involving the AstraZeneca and Pfizer vaccines were compared with the use of two doses of the same mRNA vaccine. The trial involved 340 health workers from the German capital, who were given either the combined regimen with an interval of 10 to 12 weeks between doses or conventional two-dose immunization with the Pfizer vaccine and an interval of three weeks. Three weeks after administration of the second dose, the immunological protection afforded by the combined regimen was slightly greater than the standard approach. A second German study with a similar design involving 216 Saarland University employees reached similar conclusions.
Although more time and research is needed before scientists can determine the true efficacy and long-term safety of combining two COVID-19 vaccines, the preliminary evidence for adopting this alternative immunization regimen appears to be satisfactory. According to the German media, Chancellor Angela Merkel, the country’s head of government, was advised to adopt this strategy by her medical team. For her first dose, Merkel received the AstraZeneca vaccine. For her second, she was given the Moderna vaccine, which also uses mRNA technology. Germany recommends that anyone initially immunized with the AstraZeneca vaccine receive a second dose of an mRNA vaccine.
Scientific articles
LIU, X. et al. Safety and immunogenicity report from the com-CoV study – A single-blind randomised non-inferiority trial comparing heterologous and homologous prime-boost schedules with an adenoviral vectored and mRNA Covid-19 vaccine. SSRN (preprint). June 25, 2021.
HILLUS, D. et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: A prospective cohort study. medRxiv (preprint). June 2, 2021.
BOROBIA, A. M. et al. Reactogenicity and immunogenicity of BNT162b2 in subjects having received a first dose of ChAdOx1s: Initial results of a randomised, adaptive, phase 2 trial (CombiVacS). SSRN (preprint). May 27, 2021.