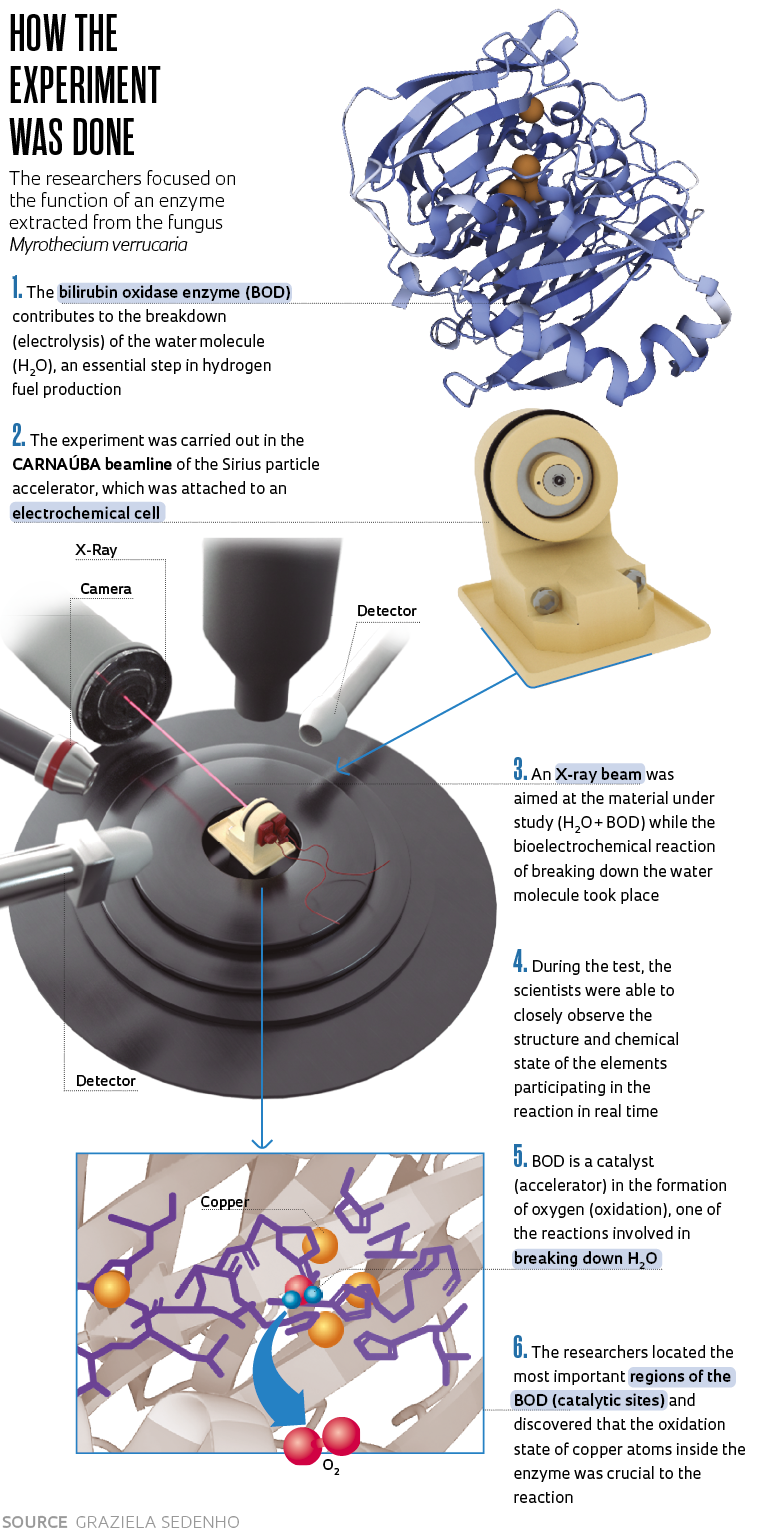
An experiment recently carried out at the Sirius synchrotron light source, based at the Brazilian Center for Research in Energy and Materials (CNPEM) in Campinas, São Paulo (see Pesquisa FAPESP issue nº 269), showed how a certain biological catalyst makes the breakdown of water molecules (H2O) via electrolysis more efficient. This reaction, an electrochemical process that uses electricity to decompose water into its constituent elements, is of great interest because in addition to oxygen, it also provides hydrogen, highlighted by many experts as the fuel of the future, since it generates no pollution (see Pesquisa FAPESP issue nº 314).
“We discovered that when manipulated in the lab, some enzymes found in nature, including bilirubin oxidase [BOD], can accelerate the water-breakdown reaction,” says chemist Frank Nelson Crespilho, a professor at the São Carlos Institute of Chemistry of the University of São Paulo (IQSC-USP) who led the study. “We didn’t know why this was happening. Thanks to new equipment developed especially for Sirius, we were able to observe how this enzyme, BOD, behaves during the water-oxidation process. We found that the copper atoms inside it play an important role in the reaction.”
Crespilho believes scientists will be inspired by the part of the enzyme that accelerated the reaction. “It is interesting that we recognized the important regions of the BOD because now synthetic chemists that produce materials can copy that part of it and synthesize it in the lab. This will make the catalyst much cheaper and greatly expand its potential applications,” says the scientist. The catalysts currently used in the process are generally made of noble metals, such as platinum and iridium, which are more expensive and thus make large-scale application unfeasible. An article describing the experiment, written by Crespilho’s team, which includes Graziela Sedenho, Rafael Colombo, Thiago Bertaglia, and Jessica Pacheco, was published in the journal Advanced Energy Materials in October. Scientists from the Brazilian Synchrotron Light Laboratory (LNLS) also participated in the study.
Researchers around the world are searching for new water-oxidation catalysts
Bilirubin oxidase was extracted from the fungus Myrothecium verrucaria, commonly found in soil and on plants. When manipulated in the lab, it contributes to the water breakdown reaction — something that does not occur spontaneously in nature. Inside the reactor, the enzyme acts more specifically on the formation of molecular oxygen, which is one of the two reactions required to break down the H2O molecule. The other is the formation of hydrogen. The two occur concurrently. “Regarding hydrogen formation, which takes place on one side of the reactor, everything is already better known. There are cheaper and more efficient catalysts. The water-oxidation reaction, however, is very slow and scientists all over the world are looking for better catalysts for this,” explains Crespilho.
The researchers were only able to observe the enzyme’s behavior during the bioelectrochemical reaction in such detail thanks to Sirius’s cutting-edge infrastructure. The team used the Tarumã experimental station of the CARNAÚBA beamline, which is still in the scientific commissioning phase, involving testing, technical development, routines, and experimental strategies.
“Various types of experiments and scientific topics are addressed in this phase, with the aim of demonstrating the beamline’s potential,” says physicist Helio Cesar Nogueira Tolentino, head of the Heterogeneous and Hierarchical Matter Division at LNLS. Of the 14 beamlines initially planned for Sirius, seven are already operational. Each operates on a different energy band, using a primary technique. All seven are open to scientists from Brazil and abroad.
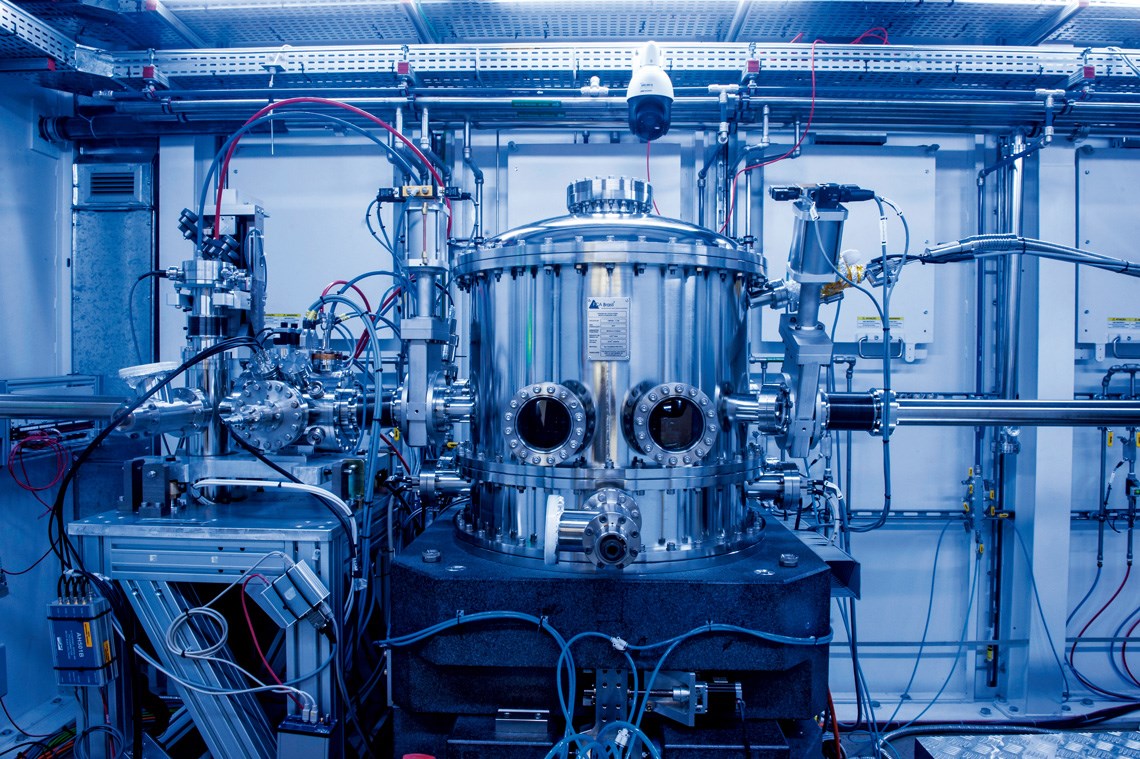
Léo Ramos Chaves / Pesquisa FAPESP Magazine Monochromator: part of the CARNAÚBA beamline at Sirius, where the study was carried outLéo Ramos Chaves / Pesquisa FAPESP Magazine
Operating since late 2021, the CARNAÚBA beamline is the longest at Sirius. It was designed for X-ray absorption spectroscopy, allowing experiments to be carried out with different materials at a nanometric scale. In addition to the powerful, superfocused beam of light, Crespilho’s group was also given access to a device recently developed by the LNLS team for the field of biochemistry.
“It is an electrochemical cell for in situ experiments. It is placed in front of the X-ray beam, which focuses on the material being studied at the moment the chemical reaction occurs. With this cell, we can also apply electrical potential and measure the current or apply the current and measure the potential, allowing us to see how the material responds to these external stimuli. All while the chemical reaction is taking place,” explains Itamar Tomio Neckel, a physicist from the CARNAÚBA group at LNLS and lead developer of the new electrochemical cell, a device small enough to fit in the palm of the hand.
The biggest challenge, according to the researcher, is to miniaturize everything, since the reactions have to take place in an extremely limited physical space. At the same time, the conditions found in the labs of different users need to be simulated. The CARNAÚBA beamline is 100 times smaller than a strand of hair and is known as an X-ray nanoprobe.