The tragic number of COVID-19 cases and deaths, which is still rising daily almost three years after the SARS-CoV-2 virus was first identified at the end of 2019, could be higher were it not for the urgency with which many governments and companies around the world stepped up to face the health crisis. Although the pandemic has limited the creation of innovative products in many sectors, the opposite has occurred in health and medicine. In record time, COVID-19 tests, medicines, and vaccines were developed, innovative equipment was manufactured for emergency care and Intensive Care Units (ICUs), and masks and coverings were produced to protect the population and reduce the spread of the virus, among other initiatives.
Some of the promising technologies designed to combat the pandemic never advanced beyond prototypes. Others were used on an emergency basis but did not take off commercially. But there are some that have successfully established themselves on the market. Pesquisa FAPESP has reported on roughly 50 of these innovations developed in Brazil since the beginning of 2020.
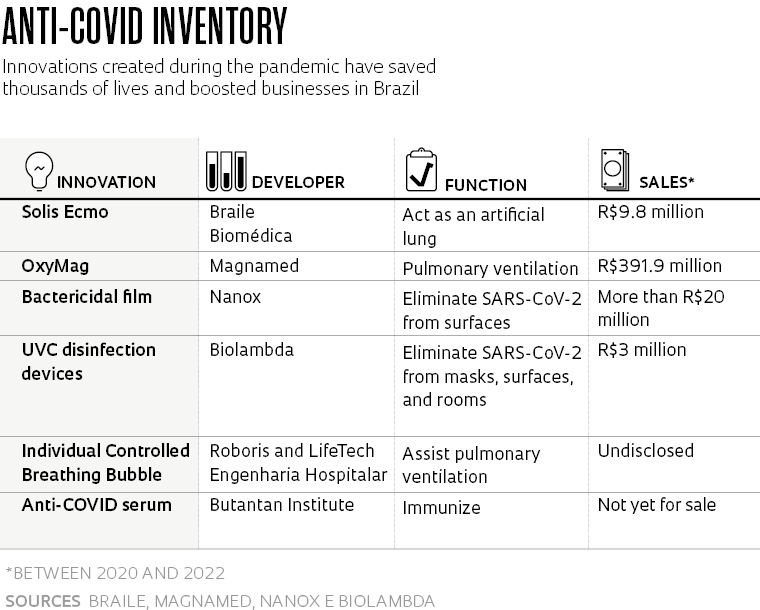
One of the most successful is the Solis System, a kind of artificial lung created by the Research, Development, and Innovation (RD&I) team at Braile Biomédica, a company based in São José do Rio Preto, São Paulo State. The device gives patients in critical condition with COVID-19 or other lung diseases a chance of survival when mechanical ventilation can no longer be used to keep them breathing.
The equipment is used for extracorporeal membrane oxygenation (ECMO), a treatment method indicated for cases of acute respiratory failure. But it can also be used temporarily as an artificial heart for patients who have had a heart attack, heart transplant, or suffered cardiac arrest.
“The machine pumps blood from the patient’s body via cannulas and tubes, adds oxygen to it using a polymeric membrane, then sends the blood back to the patient. It is an invasive, life-supporting treatment that can help keep a patient alive until their lungs recover,” explained Rafael Braile, director of operations and RD&I at the company (see Pesquisa FAPESP issue nº 301).
“Our biggest challenge now is to expand the indications for use of Solis beyond COVID-19,” says Braile. Efforts in this regard began recently. The company’s artificial lung, the first ECMO device created in the southern hemisphere according to the engineer, is now being exported and used to alleviate the effects of another crisis with global impacts: the invasion of Ukraine by Russia. “We have sold more than 20 machines to the Ukrainian government. They are bieng used in public hospitals to treat victims of smoke inhalation,” he says.
Braile is also working on a new version of the system for pediatric use. At the same time, the company is seeking to regulate the product in the European Union and the USA in order to expand its market.
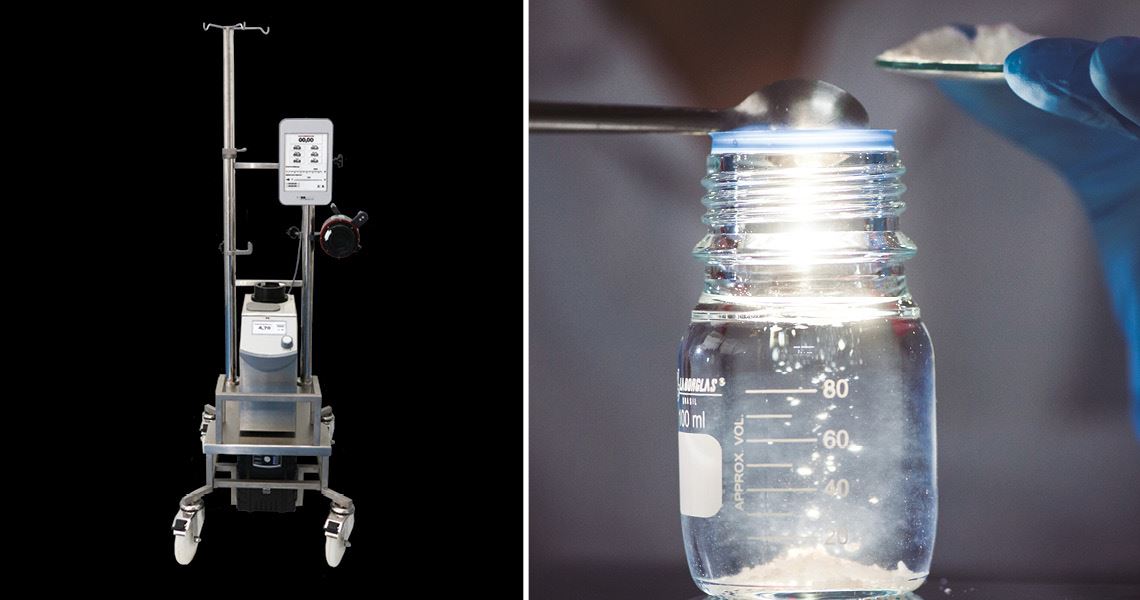
Braile Biomédica | Léo Ramos Chaves/Pesquisa FAPESP MagazineBraile Biomédica’s Solis System (left), indicated for patients with acute respiratory failure; below, Nanox antimicrobial solutionBraile Biomédica | Léo Ramos Chaves/Pesquisa FAPESP Magazine
Antiviral coatings
In July 2019, nanotechnology startup Nanox, based in São Carlos, São Paulo State, took part in an acceleration program in Silicon Valley. The accelerator in question was Plug and Play, which in the past has invested money in companies that went on to become household names, such as Google, Rappi, and Dropbox.
The American investment firm learned that Nanox had developed an antimicrobial material based on silver microparticles, which when applied to milk packaging, doubled the product’s shelf life. The innovation was the result of a project funded by FAPESP’s Innovative Research in Small Businesses (PIPE) program.
The same technology was applied almost a year later by Nanox in the fight against the pandemic caused by the novel coronavirus. In June 2020, the company launched a fabric coated with silver microparticles that inactivates SARS-CoV-2, eliminating 99.9% of the viral load within two minutes of contact (see Pesquisa FAPESP issue nº 293). The material was developed through a collaboration with scientists from the Institute of Biomedical Sciences at the University of São Paulo (ICB-USP), Jaume I University in Spain, and the Center for the Development of Functional Materials (CDMF), one of the Research, Innovation, and Dissemination Centers (RIDC) funded by FAPESP.
The company currently applies its silver microparticles—an antimicrobial active ingredient that eliminates viruses, bacteria, and fungi—to various products, including personal protective masks, professional uniforms, MDF wood used in furniture, and plastic films for frequently touched surfaces such as doorknobs, handrails, elevator buttons, and touchscreens.
“The most important sectors for Nanox are architecture and civil construction, with technological applications in paints, furniture, food packaging, and textiles,” explains Gustavo Simões, CEO of Nanox. The pandemic acted as a driving force for Nanox’s business, says the entrepreneur.
“Our biggest client has always been the industrial sector, but Brazilian industry is not known for investing in innovation. Presenting our solutions and negotiating has always been a time-consuming process.” Suddenly, he says, the pandemic accelerated everything and customers began asking themselves what they could add to their business. “The uncertainty about how some companies would survive the pandemic led to a search for innovation. And our product, in a way, met that need,” he says.
New opportunities also emerged overseas. The company currently exports its silver microparticles to customers in the USA and countries in Latin America and Europe.
Biosafety
The health emergency caused by SARS-CoV-2 also gave extra impulse to São Paulo–based startup Biolambda. Founded in 2017, it spent its first two years developing and manufacturing optical and photonic artifacts for scientific research. Shortly before the pandemic began, the company, which had already received funding from FAPESP’s PIPE program, decided to enter the microbiological control market in an industrial environment using type C ultraviolet light (UVC). This type of radiation destroys the nucleic acid of viruses and bacteria, leaving them unable to replicate and infect the body.
“Due to the pandemic, we decided to create solutions that use the same technological basis as UVC radiation to fight the coronavirus,” recalls Caetano Sabino, founder and CEO of the startup. “In less than three months, we launched a new line of equipment for efficiently disinfecting masks, surfaces, air, and spaces.” Another innovation that emerged in 2020 was a remote-controlled robot capable of disinfecting hospital rooms, clinics, and other environments.
According to the entrepreneur, the company has already sold hundreds of items from its biosafety line, named the UV Mask, UV Air, UV Room, and UV Surface. Deals for about 20 robots are also being negotiated with hospitals and clinics around Rio de Janeiro and São Paulo. “We believe that this product can increase biosafety in general in healthcare settings. In addition to working against the coronavirus, it is also effective against other viruses, bacteria, and fungi. And it can help reduce hospital-acquired infections caused by different microorganisms,” says Sabino.
The biosafety line currently accounts for around 60% of Biolambda’s revenue, which was R$2.5 million last year. “The pandemic greatly accelerated the development of our innovations and had a positive impact on the company’s income,” says Sabino, who celebrated the startup’s first export contract to the UK in August. It had already begun selling its UVC disinfection products to customers across Latin America.
Vital ventilators
São Paulo–based Magnamed, the largest manufacturer of lung ventilators in Brazil, saw demand for its products soar due to the pandemic—the devices are used to help patients who cannot breathe by themselves. To scale up production, the company’s CEO, electronic engineer Wataru Ueda, utilized his network of contacts from the Technological Institute of Aeronautics (ITA), where he studied his degree, to obtain investments that would allow the company to increase production. As a result, leaders of major Brazilian companies, such as pulp and paper manufacturer Suzano and aeronautical company Embraer, both part of Ueda’s network, joined the effort to manufacture more equipment for the health system.
Thanks to the financial reinforcement, Magnamed was able to increase production and, in the first year of the pandemic, agreed a deal to supply 6,500 ventilators to the Brazilian Ministry of Health. The contract led to record revenues of R$340 million that year, 7.5 times higher than in 2019. With the domestic market well supplied in terms of the number of respirators in hospitals, the company began planning to go international.
Magnamed wants 80% of its revenue to come from exports by 2026—they currently account for about a third of the total. The company’s globalization process includes construction of plants to manufacture and assemble the devices abroad. The first, located in Miami, USA, will produce the OxyMag electronic ventilator, Magnamed’s flagship product. The company is awaiting approval from the US Food and Drug Administration (FDA) before it starts producing the device.

BiolambdaRobot and device created by Biolambda to disinfect rooms and surfaces respectively using UVC raysBiolambda
During the early months of the pandemic, universities and companies around Brazil began working on assisted breathing devices capable of facilitating lung ventilation. Similar in appearance to a diving helmet, these machines help minimize the inflammatory effects of the viral infection. They can prevent the need for intubation in hospital patients, which is seen as the last resort in acute COVID-19 cases.
One such device is the Individual Controlled Breathing Bubble, created by technology company Roboris and sold by LifeTech Engenharia Hospitalar, both based in São Paulo. The product is approved by the Brazillian Health Regulatory Agency (ANVISA) and has already been used by more than 4,000 patients in 100 hospitals in 15 states since the beginning of 2020.
“At that crucial moment of the pandemic, when there were no beds left in the ICUs and Brazil was struggling to care for critical COVID-19 patients, the Individual Controlled Breathing Bubble was an important option, reducing the need for invasive mechanical ventilation [intubation],” recalls Guilherme Thiago de Souza, general director at LifeTech.
The seed for creation of the company’s assisted ventilation device was planted within the Brazilian Aerospace Cluster, a group of aerospace companies based in the São José dos Campos Technological Park in São Paulo State. It was there that in the first half of 2020, a team of engineers had the idea of combining the theory of noninvasive ventilation with the helmet concept, which emerged in other countries in the 1980s but was still unavailable in Brazil.
After facing difficulty finding components for the helmet, Souza turned to creative and constructive engineering processes to make the Individual Controlled Breathing Bubble industrially and commercially viable. The first prototype was ready in 40 days. The device was adopted for use in ICUs, where the mortality rate of intubated COVID-19 patients was around 80%.
“Because of the success of our ventilation helmet, the Department of Pulmonology invited me to do a PhD at the School of Medicine of the University of São Paulo [FM-USP],” says Souza. For his doctorate he is working on a new project related to airways.
The São Paulo–based institute is developing a product from samples of SARS-CoV-2 inactivated by radiation
The Butantan Institute, based in São Paulo, played an important role in the development of vaccines to prevent the most serious effects of COVID-19. In addition to its partnership with Chinese pharmaceutical company Sinovac, under which it bottled and later produced CoronaVac, the first COVID-19 vaccine applied in Brazil, Butantan also invested in the development of an anti-SARS-CoV-2 serum. Once approved by health authorities, it could be used to stop the spread of infection in people with early symptoms.
The serum, made from samples of the coronavirus inactivated by radiation and administered to horses, took five months to prepare. The horses’ bodies responded to the presence of the virus by producing immunoglobulin G (IgG) antibodies, which were then extracted from the blood and purified, creating a concentrated blend of specific antibodies called a serum. After satisfactory results in testing on mice and rabbits, the serum was tested on humans, with authorization from ANVISA.
The initial protocol was for phase 1 and 2 clinical trials in three stages. The first stage of phase 1, which focused on product safety and dosage, was successfully completed at Hospital do Rim in São Paulo in January, involving 30 patients who had contracted COVID-19 after having a kidney transplant. The serum was well tolerated and allowed the researchers to define the ideal dose for the remainder of the trials.
The second stage of phase 1 was set to involve 30 patients with solid organ cancers from the Hospital das Clínicas at FM-USP, while phase 2 would have included 558 individuals, including people who had undergone solid organ transplants and cancer patients, all undergoing immunosuppressive therapy.
“When the profile of the epidemic changed shortly before the start of the trial with cancer patients, with a fall in severe COVID-19 cases and the rise of the omicron variant, we decided to redefine the protocol to expand the use of the serum and to also treat immunocompetent people [capable of reacting to microorganisms by producing antibodies] in a greater number of locations,” explains biochemist Ana Marisa Chudzinski-Tavassi, director of innovation at the institute. “At the same time, several studies have shown that the serum is also capable of neutralizing this strain [omicron].”
The scientist notes that the Butantan Institute has been working to strengthen its existing structures and establish new platforms for technological innovation. “FAPESP even recently approved a project of ours as part of the Science for Development Centers [CDC] program. It includes a series of facilities needed for developing new products, including mRNA vaccines and recombinant proteins,” she says. “This financial support is important for us to continue developing our own technologies and strengthening the country’s innovation ecosystem.”
Projects
1. Development and preclinical validation of stent-graft endoprostheisis and catheter for treatment of peripheral vascular diseases (nº 15/50608-0); Grant Mechanism Innovative Research in Small Businesses (PIPE); Agreement FINEP – PIPE/PAPPE Grant; Principal Investigator Domingo Marcolino Braile (Braile Biomédica); Investment R$744,000.
2. Synthesis and application of nanostructured fungicidal materials (nº 15/50113-1); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Luiz Gustavo Pagotto Simões (NANOX); Investment R$734,552.36.
3. Electronic pulmonary ventilators on emergency transport (nº 09/52278-7); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Wataru Ueda (Magnamed); Investment R$135,576.27.
4. Development of smart equipment for clinical application of phototherapies with automatic dosimetric adjustment and payment on demand (nº 17/22406-0); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Caetano Sabino; Investment R$189,458.65.
5. Continuous improvement of vaccines: Center for Viral Surveillance and Serological Assessment (CEVIVAS) (nº 21/11944-6); Grant Mechanism Science for Development Centers (CDC); Principal Investigator Sandra Sampaio Vessoni (Butantan); Investment R$4,826,106.75.