In 1977, upon entering the School of Medicine at the Federal University of Pará, Pedro Vasconcelos planned to become a cardiac surgeon. He gave it up when he watched an actual surgery and concluded that he wouldn’t be able to do anything of the sort. During his fourth year of medical school he began a virology internship at the Evandro Chagas Institute (IEC), an NGO linked to the Brazilian Ministry of Health and one of the most important centers for tropical disease research in the country, and he never left the subject. He began his research under the guidance of virologist Amélia Paes de Andrade Travassos da Rosa (1937–2017), who, two decades later, would again supervise him during his postdoctoral studies at the University of Texas Medical Branch (UTMB), in the United States. Hired by the IEC shortly after graduating in 1983, he helped diagnose the first cases of dengue in Brazil, in Boa Vista, Roraima State. He subsequently specialized in the study of arboviruses, the insect-borne viruses such as yellow fever and Zika fever. In this interview, given in August, he affirms that both diseases will return to put the country on edge once again.
In 2015, his research group at IEC isolated the Zika virus from the brain of a microcephalic infant, the son of a woman in the state of Ceará who became infected during pregnancy. Based on this work, the Ministry of Health issued a statement establishing an association between Zika infection and microcephaly. The following year, the epidemic swept the Americas and Vasconcelos represented Brazil on the emergency committee of the World Health Organization (WHO) that was addressing the issue.
Specialty
Virology
Education
Undergraduate degree in medicine from the Federal University of Pará (1982), specialization in tropical medicine from the University of São Paulo (1987), and PhD from the Federal University of Bahia (1999)
Institution
Evandro Chagas Institute
Scientific production
Close to 280 scientific articles, 62 book chapters, and 6 published books. Supervised 32 master’s theses and 21 doctoral dissertations
Born in Monte Alegre, 600 kilometers from Belém, Vasconcelos is 61 years old and is married to Helena Baldez, with whom he has three children: Pedro, a prosecuting attorney; Beatriz, a physiotherapist; and Barbara, a doctor.
Over almost four decades, Vasconcelos has participated in identifying and describing more than 100 virus species. Director of the IEC since 2014, he is part of a research group that explained the action of yellow fever virus in monkeys, facilitating the understanding of the phenomena observed in people afflicted with this disease, which still has no cure.
Will yellow fever come back?
It is likely, especially in São Paulo, Rio de Janeiro, and Paraná, because of how widespread the virus is in these states. Even during the winter there were reports of monkey deaths from yellow fever on the São Paulo coast, a sign that the virus continues to spread. Vaccination coverage was less than expected in some areas of these states, which previously hadn’t seen incidences of wild yellow fever. Now the entire country is receiving the vaccine, even in the Northeast, where vaccination wasn’t mandatory.
Should everyone in the country be vaccinated?
Vaccination should be done with caution, but in my view, the entire country needs to be vaccinated. I’ve been arguing for this since the outbreaks in Minas Gerais in the 1990s. It had been the plan to make the vaccination universal by the year 2000. That was the intention of the technical advisory committee of the National Immunization Program and the then Minister of Health, José Serra. Then the first two fatal cases of acute viscerotropic disease occurred as a reaction to the vaccine, which put a halt to the vaccination plan. Until then the yellow fever vaccine had a pristine image, nobody had anything against it. Viscerotropic disease is identical to naturally acquired yellow fever. The difference is that in the first case we see a very large quantity of virus in the blood, kidneys, heart, and spleen of the people who die from it. It’s estimated that there’s one case of acute viscerotropic disease for every 500,000 to 1 million people vaccinated.
How is your research on yellow fever going?
With a doctoral student, Milene Silveira Ferreira, I’m concluding a study on the evolution of the disease in monkeys of the genus Saimiri, the black squirrel monkey, to understand why a healthy patient suddenly worsens and dies. We gave the monkeys an intradermal infection simulating a mosquito bite, and we saw that the dendritic cells receive the virus and take it to the nearest lymph node [small organs that participate in the body’s defense against disease-causing agents]. There, the virus is introduced to the CD4 T lymphocytes and replicates inside them, causing them to rupture and release thousands of copies of the virus. These copies land in the lymphatic system and the bloodstream. This is primary viremia, which coincides with the febrile period and occurs during the first five days. Then the virus goes to its target organs. The principal organ is the liver, but the spleen, heart, and kidneys are also affected. In the kidney, acute tubular necrosis occurs, leading to decreased—or even the cessation of—urination. In the heart, there is muscle-cell death, which, perhaps, is responsible for sudden deaths in the late stage of the disease. In the liver, Kupffer cells, which protect against microorganisms, are killed, as well as hepatocytes, which are responsible for most liver functions. The damage to the monkeys’ livers looks more like that of fulminant hepatitis. It’s more extensive than the damage observed in cases of yellow fever in humans.
What happened to the monkeys in your experiment?
Only one of ten that were infected died. We’re investigating 30 immune system markers, including cells, cytokines, and chemokines [molecules that activate immune cells], to identify something that signals whether the animal will worsen and die, or recover. The viremia remains high until the fifth day and then drops dramatically. On the sixth day, the liver damage intensifies, when, paradoxically, the concentration of virus in the blood decreases and antibodies begin to appear. This deterioration is probably due to the action of immune cells and the release of cytokines and chemokines. This also happens in people who develop the severe form of the disease and die between the seventh and tenth day. In the animals, we didn’t see brain injury, which is relatively common in humans. What we observed in the tissue analysis has been known since the late nineteenth century, but we have a lot of new data about the immune response. For example, we didn’t know the extent of the compromise to the vascular endothelium in the liver, which is quite severe, and may be linked to the activation of cytokines and chemokines. The involvement of bile duct cells, which express viral antigens, was also unknown. This phenomenon is very clear in dengue and Zika. In two recently published articles, we’ve shown that in the children with microcephaly who died, the death of cells in the nervous system activates cytokines and a set of proteins called inflammasome. We’ve observed that inflammasome induces intense destruction in the infected areas of the brain.
How does one explain the return of measles and other diseases that had been under control?
The difficulty of controlling epidemics of older diseases, such as measles or dengue, or preventing the introduction of viruses originating from other places into Brazil, such as Chikungunya and Zika, has several causes. One is the increasingly intense and rapid movement of people from one side of the planet to the other. This is a powerful mechanism for the spread of disease, which can’t be prevented. In Brazil, where the healthcare structure and disease monitoring system is still flawed and passive, it’s almost impossible. An additional difficulty is the low educational level of the population, which facilitates the spread of diseases because people avoid seeking health care, which could lead to identifying cases earlier. One example of this was the chikungunya epidemic. Everyone knew that it was coming, and when it appeared, the country didn’t have the speed and efficiency to control the two outbreaks, which began simultaneously in Bahia and Amapá.
Why wasn’t there a faster response?
I see a disconnect between the federal, state, and municipal governments. The Unified Health System, SUS, is one of the best organized in the world, but it would have to compensate for these deficiencies, which are chronic in most states. São Paulo has a more organized health system. In Amazonia, states face difficulties because of the distances involved and a lack of infrastructure. The states of Pará and Amazonas are huge, totaling about 30% of the Brazilian territory, with regions that can only be reached by helicopter, plane, or boat. Monitoring and vaccination are difficult to do in these states. In addition, the staffs at public health agencies are substantially smaller. At the Evandro Chagas Institute, the last hiring competition was in 2010, when we were about to collapse due to a lack of employees, and currently a lot of our people have already been working long enough to retire.

Léo Ramos Chaves
People line up to get vaccinated against yellow fever in the city of São Paulo in January 2018Léo Ramos ChavesYour group was the first to isolate the Zika virus during the recent epidemic. How did that come about?
We isolated it first, but Gúbio Campos, a professor at the Federal University of Bahia was the first to describe it, using molecular biology, and show that Zika was responsible for the cases of febrile disease in that state. We were investigating this possibility due to cases that had occurred in Rio Grande do Norte. At the end of February 2015, Professor Kleber Luz, an infectious disease specialist at the Federal University of Rio Grande do Norte called me, describing strange cases in the area that he didn’t think could be dengue, and asked if he could send samples to examine in Belém. They sent them and it wasn’t dengue. Nor was it chikungunya, which was equally prevalent. He suspected it might be Zika, but we were out of reagents for doing the tests. Gúbio had gotten his doctorate in Argentina and had reagents for a lot of viruses. In early April, he released the result. It took two weeks. After the reagents arrived at our lab, the Ministry of Health asked if we had the capacity to do testing and I replied, “We do now.” In two weeks, we isolated the virus from a patient from Paraíba and gave it to other groups. Since we’re a national reference laboratory and we collaborate with the Pan American Health Organization, OPAS, and the World Health Organization, WHO, we sent samples to FIOCRUZ [Oswaldo Cruz Foundation] in both Rio and Minas, to UFMG [Federal University of Minas Gerais], to USP [University of São Paulo], and to Singapore, Australia, South Korea, and the United States. Some beautiful work came out as a result. Later, we also isolated it from a baby in Ceará, which allowed us to show, for the first time, the connection between microcephaly and the brain lesions caused by the virus, in late November 2015. Based on this research, the ministry released a statement confirming the association between Zika and microcephaly. This was before a study was published in the New England Journal of Medicine in which a team from Slovenia analyzed tissue from a baby, the son of a woman who became pregnant during a vacation in Natal, Rio Grande do Norte. She had a surgical abortion in the eighth month of gestation, which is allowed in that country in the case of microcephaly.
Was there any dispute regarding the conclusion that the Zika virus could cause microcephaly?
There were those who said microcephaly was caused by the use of expired vaccines, insecticides, and other things that were unproven. At the end of October that year, the Ministry of Health gathered all the principal players involved in the investigation in a hotel in Brasília. They were clinicians from Pernambuco, Ceará, Rio Grande do Norte, São Paulo, Rio de Janeiro, and other states. We wanted to come to a conclusion on what action to take. Every day the number of cases increased and there was no conclusion about the causes. I said, “If you give me good, well-preserved clinical material, I will quickly make the diagnosis.” Fernanda Montenegro de Carvalho Araújo, a virologist at the Central Laboratory of Public Health of Ceará was there, and told me she would send the material. She sent samples collected from a baby on November 18. An autopsy had been performed and we had tissue samples from the brain, spleen, kidney, heart, lung, and blood from the umbilical cord. We replicated the viral material from all the tissues, did a pathological analysis, and cultivated the virus. The child’s mother had had what appeared to be dengue or an allergy around the eighth week of pregnancy. She had gone into labor and, as the baby didn’t come out naturally, a cesarean section was done. The baby had a series of malformations and died shortly after birth. We did the tests and they were positive for Zika. The virus was found in every organ. We wrote a report on November 28, and the Ministry of Health and OPAS published it on December 1, 2015.
Why did the Zika virus mainly affect poor women in the Northeast?
There are a number of factors. The Northeast has always been the region with the highest number of reported cases of dengue, except when epidemics break out in more populous states such as Minas, Rio, or São Paulo. This is associated with a higher level of mosquito infestation. If there are more mosquitos, there’s more of a breeding ground. A virus that arrives there multiplies exponentially, which may have happened with Zika. The virus is found where there are more mosquitos, which is the urban periphery, due to a lack of basic sanitation. What surprises me is that we have basically the same conditions regarding the lack of sanitation on the periphery of large cities in other regions where there were fewer cases. Together with virologist Maurício Nogueira, from FAMERP [Medical School of São José do Rio Preto, in the state of São Paulo], I participated in the evaluation of a population monitoring study in São José do Rio Preto, where there was almost no microcephaly. Consuelo Oliveira, a pediatrician and IEC researcher, tracked two cases of microcephaly in Belém, where more than 150 women became infected with Zika during pregnancy and had babies born without microcephaly, although other deficiencies such as learning disabilities and deafness are emerging.
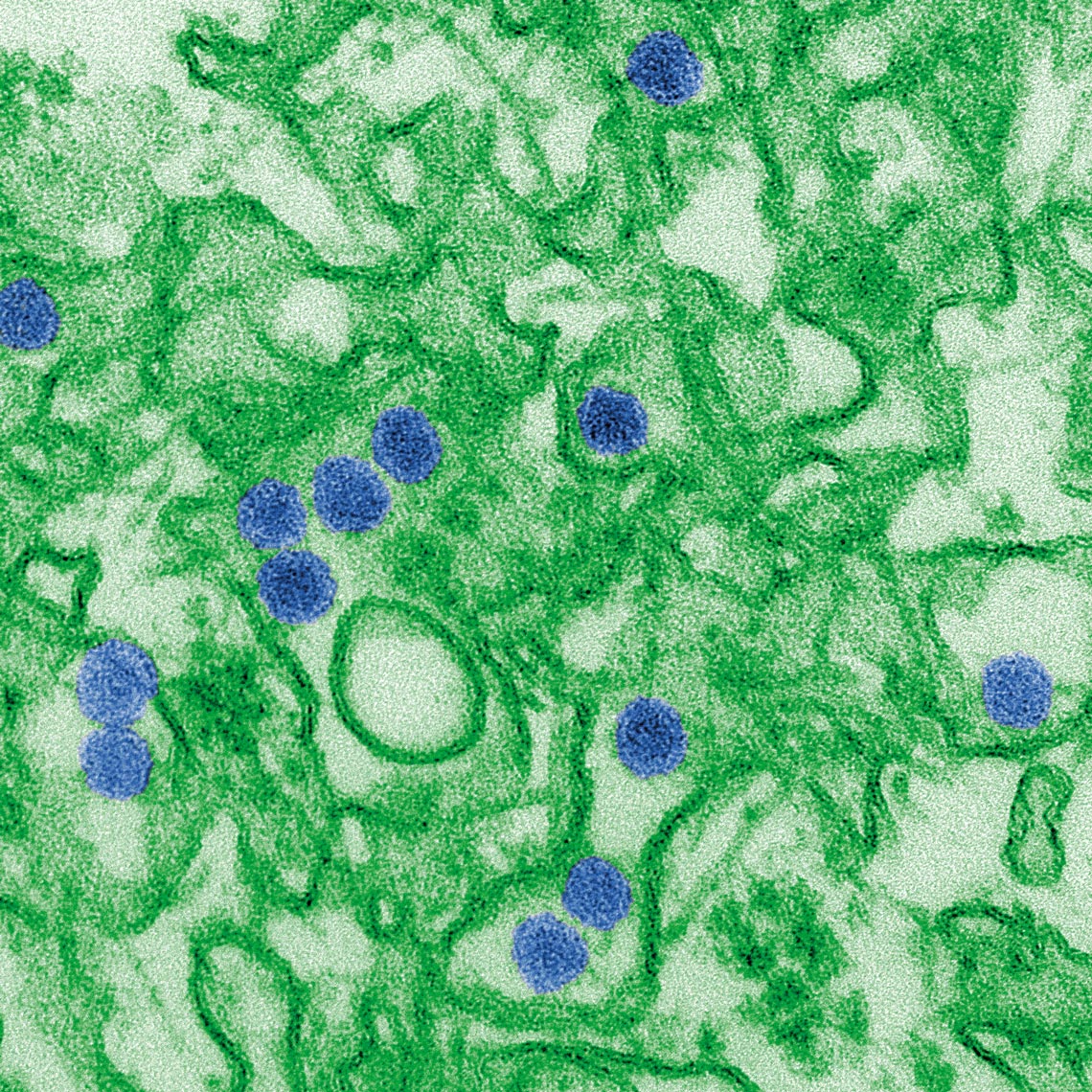
Cynthia Goldsmith / CDC
Artificially colored electron microscopy image shows copies of Zika virus (in blue)Cynthia Goldsmith / CDCIs help for children born with microcephaly caused by Zika a problem today?
There is no doubt that the country hasn’t given all the support it should. There was a lot done at the beginning; the decision of then president Dilma Rousseff to free up the money to create the emergency fund for supporting research was important. Ricardo Barros, who was a congressman before becoming health minister, approved a parliamentary amendment of R$500 million to combat Zika, but the money ran out. These children will need support for their entire lives. They need physiotherapy, occupational therapy, speech therapy, and medical assistance from neurologists, pediatricians, and other specialists. I’ve seen heartbreaking documentaries showing that there is still major upheaval in these families. Many men abandoned their wives and sick children. These people need government support, especially from local government.
How is the Zika vaccine coming?
My commitment to the then Minister of Health, Marcelo Castro, was to develop the vaccine, but not to participate in clinical trials, which isn’t my area. In December 2015, Castro and later José Agenor Álvares Silva, then executive secretary, asked if we had the capacity to produce a vaccine at our institute. “We do,” I said. “So, are you up for coordinating it?” they asked. We said yes, as long as we could partner with a European or American institution, in order to speed up the production of the vaccine. There isn’t an institution in Brazil that would be able to do it quickly. Working with an American institution, we could get it done in a year. We actually did it in less than a year. At the meeting I attended in Manaus when I received the request from the Ministry, there were also people from the UTMB [University of Texas Medical Branch], the NIH [National Institutes of Health], and the CDC [Centers for Disease Control and Prevention]. I spoke with Robert Tesh (see Pesquisa FAPESP issue no. 257), a virologist at UTMB, where I had done a postdoc. At the time he was a Special Visiting Researcher in Brazil, brought in by the federal government. I asked if the University of Texas would be interested and he said yes, and that there were two other researchers who might be interested: Scott Weaver, who was at the Manaus event, and Pei-Yong Shi. Scott said he already had a lot of commitments and thought Pei-Yong would be better. I spoke with the Chinese researcher and we scheduled a technical visit to Texas, which took place in January 2016. With Daniele Medeiros and Bruno Tardelli of the IEC, we established the Cooperation Agreement with two employees from the Ministry of Health. In February, the Minister signed it and later the funds were transferred, via OPAS, from Brazil to UTMB. Bruno and Daniele spent a year and a half there developing a vaccine. We started the research at the end of April and in October we already had the initial results from the chimeric viruses, which would be the strongest candidates for vaccine production. The paper was published in Nature Medicine in January 2017. Then we did other experiments, published in Cell, and in Nature Communications, showing protection in pregnant mice. Finally, this year we published a fourth article in Cell Host & Microbe. In it, we showed that an alternative vaccine given to pregnant mice was able to protect the fetuses from the lesions caused by Zika. That concluded our part and we passed on the prototypes to FIOCRUZ. There was an agreement between FIOCRUZ, the Ministry of Health, and UTMB so that the transfer of the vaccine-production technology would be formalized. Our expectation was that clinical trials would begin in 2018, but they haven’t started. The vaccine development cost the equivalent of about R$10 million at the time. The estimate for clinical trials and production of the first lots is between R$80 million and R$120 million.
Does the country have the money and capacity to make this vaccine?
The Ministry has guaranteed the resources. There’s already been a transfer of US$2 million to pay the first installment for hiring a company capable of following the protocols for good manufacturing practices, which is required for products for human use. A transfer of around R$20 million was being negotiated to begin the production of the first lots. There are other possible vaccine compounds under development, such as a DNA vaccine, but the response was not what was hoped for. Ours seems to have the best response, although it presents a greater risk of causing problems in pregnant women. We have always said that the vaccine is not for pregnant women. It is to be applied before the age of fertility, in children under 12 years old. I have no doubt that Zika will be back and cause another epidemic. As the Zika virus has only one serotype, the expectation is that another episode will occur in five to ten years. Two years have already gone by since the first outbreak. Certainly the impact won’t be the same as the previous outbreak, but many problems could arise, especially if there’s an epidemic in less affected areas in the Northeast or in other regions. It’s just a matter of time.
Mayaro and Oropouche are the two Amazonian viruses which pose the highest risk for humans
Is the Amazon the largest natural reservoir of viruses in Brazil?
I have no doubt, and few people know this, but the Amazon is the world’s largest reservoir of arboviruses, the viruses transmitted by hematophagous insects [that feed on blood]. There is an abundance of hematophagous arthropods, which function as vectors, and intermediate hosts, mainly mammals and birds, which can serve as primary hosts for these viruses. At Evandro Chagas, we’ve isolated and described 220 arboviruses from the Amazon. One of our lines of research is to study the infection caused by these viruses, through experiments in rodents, to discover which would pose a risk of causing disease in humans. Amazonian viruses are in balance with the environment and the risk of their spreading is small, but deforestation and other human action on the ecosystem can facilitate the spread of viruses to inhabited areas and transmission to humans. It’s best not to mess with them.
Which arboviruses have already been recognized as potentially dangerous to humans?
Two viruses with a high potential to cause disease in humans are Mayaro and Oropouche. When there is deforestation and people form villages in the forest, there are usually outbreaks and epidemics, mainly of Oropouche. A colleague, Raimunda do Socorro Azevedo, is putting together data showing a silent outbreak in the metropolitan region of Belém, masked by epidemics of dengue, Zika, and chikungunya. The transmission vector of Oropouche is the insect Culicoides paraensis, known as maruim [a species of midge], which has been found in Bahia and here in the south, although not infected. The danger is that the virus would be carried by a patient in the viremic phase to a region with maruim, initiating a local transmission and exploding there. Experimentally, we know that Oropouche can infect the mosquito Aedes aegypti. It is possible that in the future, urbanization of this virus will occur by classical vectors, such as Aedes aegypti and A. albopictus, and not by Culicoides. In addition to the Oropouche, the Mayaro virus, which is transmitted by the mosquito Haemagogus janthinomys, a transmitter of wild yellow fever, has caused outbreaks in recently deforested areas. With the exception of these two viruses, however, the spread of Amazonian viruses is limited. The viruses associated with epidemics are generally exotic, such as yellow fever, which came from Africa, and more recently dengue, chikungunya, and Zika, which also originated there, and were carried from one side to the other by infected people traveling by air. Recently, the West Nile virus was introduced in Brazil. In 2015, we described a human case of encephalitis caused by this virus in Piauí, and we later isolated the virus from horses with encephalitis in Espírito Santo. Personally, I think the West Nile virus’s biggest potential is to cause a veterinary disease. There’s also the Saint Louis encephalitis virus that occurs throughout North and South America, which caused encephalitis deaths in the 1920s and 1930s during an outbreak in the city of Saint Louis, Missouri, in the United States. We did genetic studies and found that its potential to cause serious illness is immense. In Brazil, we don’t have reports of human encephalitis from this virus, but monitoring here is poor compared to the United States and Canada. At FAMERP, Maurício Nogueira described cases of meningitis caused by the Saint Louis encephalitis virus during the dengue epidemic. Exotic viruses have generated far more serious problems than the Amazonian varieties, but I do not think the latter are less aggressive. Understanding how these viruses act and distribute themselves in the Amazonian ecosystem are our priorities. Only the IEC has samples of these viruses, as well as the conditions for developing studies in their natural environments in order to understand their transmission cycles, and to perform laboratory tests with animals in order to understand how they cause disease.
