OHSU
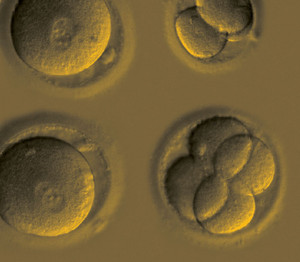
Embryos in the early stages of multiplication after gene repair via CRISPR-Cas9OHSUResearchers led by geneticist Shoukhrat Mitalipov of the Oregon Health and Science University in the United States have used a gene-editing technique to correct a mutation responsible for the late development of heart disease in human embryos. This is the first example in the United States of the elimination of a defective copy of a gene and its replacement with a complete version in the cells of the embryo with no apparent harm to its development. Using the same technique in March of this year, a team led by Jianqiao Liu from Guangzhou Medical University in China had already restored two genes associated with two forms of anemia in human embryos, but with a lower rate of success.
In the study published on August 2 in Nature, Mitalipov and 30 other researchers from the United States, South Korea, and China used a gene-editing technique called CRISPR-Cas9 to eliminate a mutated copy of the MYBPC3 gene, which encodes a protein discovered in the 1980s by Brazilian biologist Fernando Reinach. This editing system consists of a protein (Cas9) attached to a molecule that directs it to a region of DNA repeats known as CRISPR (see Pesquisa FAPESP, issue No. 240). Cas9 cuts double-stranded DNA and, in embryos, activates the repair mechanisms that produce an integral copy of MYBPC3—human cells have two, but the mutation of one causes problems. Before Mitalipov and Liu’s teams completed their studies, other groups in China tried to use this technique to edit human embryos, albeit unsuccessfully.
Mitalipov and his group were able to increase the efficacy and safety of the technique by identifying the most appropriate method and moment for its use. They injected Cas9 into the ovum with the spermatozoid at fertilization; even so, the technique worked in only half of the cases. When Cas9 was inserted after fertilization, the embryos exhibited a problem known as mosaicism: half of their cells had the corrected gene, and half had the defective version.
Initially, the researchers suspected that, while Cas9 had corrected the embryo problem, it had affected 15 more regions than expected. Further analysis found no defects in these regions, suggesting that the problem was in the verification technique used. No embryos were implanted in women, as this step is not allowed in the United States.
As Nerges Winblad and Fredrik Lanner of the Karolinska Institute in Sweden describe in Nature, this research paves the way for the clinical use of therapies based on this tool. It also raises ethical questions. It is feared, for example, that gene editing can be used in an attempt to produce stronger or more intelligent people.
“CRISPR-Cas9 is a powerful technique that can correct a mutation,” says biologist Ângela Saito, a researcher from the Genome Modification Laboratory within the Brazilian Biosciences National Laboratory (LMG-LNBio) in Campinas. “Because there is a risk of nonspecific changes and undesirable outcomes, more studies need to be done before this technique can be used to treat hereditary diseases in humans.” According to embryologist José Xavier Neto, coordinator of LMG-LNBio, “gene editing seems to present a solution to a problem that can still be solved more safely with the selection of embryos obtained via in vitro fertilization before implantation in the uterus.”
Scientific article
MA, H., et al. Correction of a pathogenic gene mutation in human embryos. Nature. Aug. 2, 2017.