Brazil is one of the best-positioned countries when it comes to large-scale production of low-carbon hydrogen, a fuel with a high calorific value that has been identified as an important vector for the transition to green energy. The country has the technical potential to generate 1.8 gigatons of hydrogen per year, with around 90% of this volume using renewable energy. The data is part of the 2031 Ten-Year Energy Expansion Plan by the Brazilian Power Research Company (EPE), linked to the Ministry of Mines and Energy (MME).
The plan identifies several sources and technological pathways for the production of low-carbon hydrogen, considered by many experts to be the fuel of the future due to its ability to help decarbonize the planet. It is expected to replace fossil fuels in sectors of the economy such as transport and energy-intensive industries (steel, metallurgy, cement, for example). The burning of fossil fuels causes the emission of greenhouse gases (GHGs) associated with global warming and climate change.
Low-carbon hydrogen is the new terminology used by the International Energy Agency (IEA) for hydrogen (H2) produced by methods that emit zero or close to zero carbon dioxide (CO2). This includes hydrogen produced from reformed ethanol and other biofuels or biomass (such as agricultural or forestry waste); from water electrolysis using renewable (wind, solar, hydraulic) or nuclear energy; from the thermal reforming of natural gas with carbon capture, utilization, and storage (CCUS); and natural hydrogen, which can be extracted from the soil.
According to the federal government’s National Hydrogen Program (PNH2), the low-carbon hydrogen projects already announced in Brazil total around US$30 billion. Energy sector analysts and scientists consulted by Pesquisa FAPESP are optimistic about the country’s leading role in this new market. “Brazil meets the conditions needed to be one of the global leaders in the sector,” says renewable energy specialist Ricardo Ruther, a professor at the Federal University of Santa Catarina (UFSC) and head of an experimental green hydrogen (GH2) production plant—where the fuel is produced through water electrolysis—recently opened by the institution.
“We have an abundance of renewable sources of wind and solar energy, which are essential for sustainable hydrogen production; an organized, competitive, and dynamic market for electricity generation from renewable sources; the industrial infrastructure needed to produce hydrogen on a large scale; and relative proximity to the European market, to which the fuel will be exported,” he states.
Renewable, clean fuel
The importance being placed on hydrogen fuel is due to the fact that its calorific value is around three times higher than that of natural gas, gasoline, or diesel. Although it is abundant in the Universe, hydrogen is rarely found in isolation. It is present, however, in ethanol (C2H6O), methane (CH4), and other fossil fuels, in addition to water (H2O). To isolate the hydrogen molecule for use as an energy source to power motor vehicles or industrial procedures, these compounds have to undergo chemical processes.
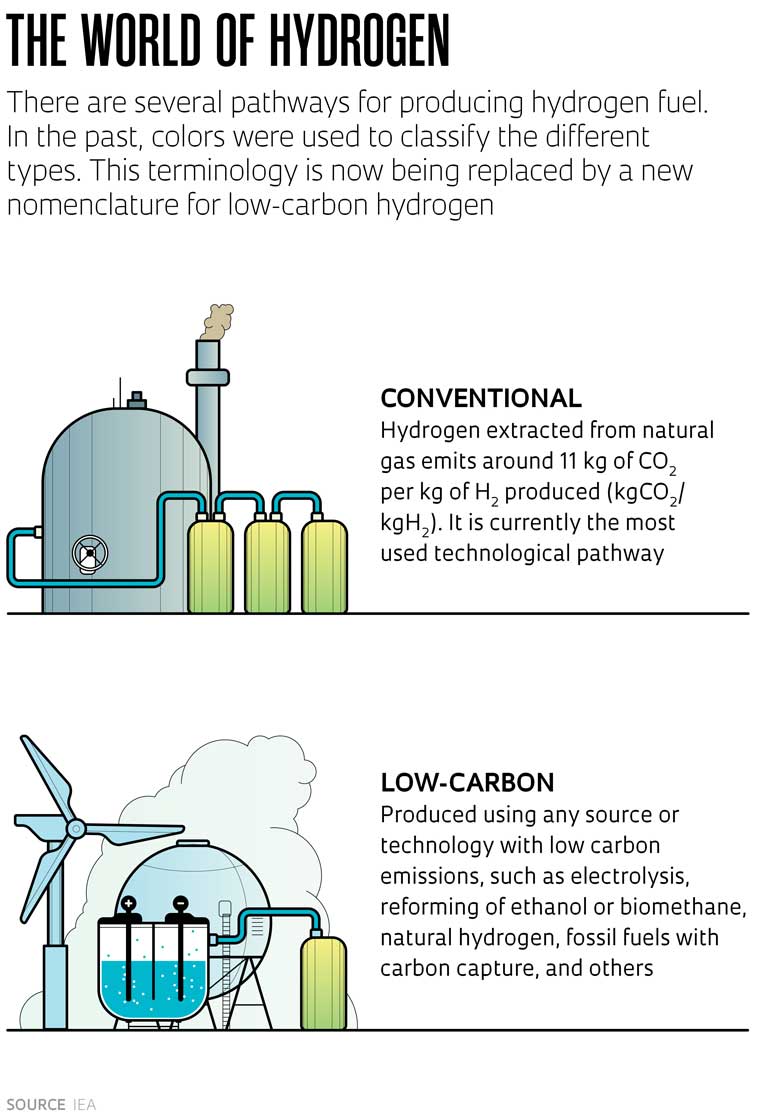
The main pathway for hydrogen production is the steam reforming of natural gas, whose main constituent element is methane. The process involves subjecting methane to high temperatures in a reactor, transforming it into H2 and CO2. The resulting hydrogen is not classified as sustainable because CO2 is released into the atmosphere during the process, contributing to the increase in GHGs. For every kilogram of hydrogen (kgH2) produced, around 11 kg of CO2 are emitted (kgCO2).
The various pathways for hydrogen production are commonly identified by colors, which can vary depending on the author. Usually, hydrogen produced from methane in a process that creates CO2 emissions is called gray hydrogen, but if the CO2 is captured, it is called blue; hydrogen produced by electrolysis is called green; and hydrogen produced using nuclear energy is called purple or pink.
Aware that this color classification is imprecise and has no practical application in decision-making processes for contracting in the sector, as well as the potential for regulatory problems, the IEA recently proposed a new nomenclature based on the level of emissions in hydrogen production (see sidebar, left). This new nomenclature includes the term “low-emission hydrogen,” which Brazil’s PNH2 program has adopted.
In Europe, for hydrogen to receive the low-emission label, the production process must generate a maximum of 3.8 kg of CO2 per kg of H2 (kgCO2/kgH2). Brazil has not yet defined an emissions limit, but a bill currently under consideration proposes a value of 4 kgCO2/kgH2.

VCG / VCG via Getty Images
Storage tanks at China’s largest green hydrogen refinery in the northwestern city of KuqaVCG / VCG via Getty ImagesGlobal production of low-carbon hydrogen is still modest. The IEA’s “Global Hydrogen Review 2023” reports that 95 million tons (Mt) of hydrogen of all types were produced in 2022. Of this total, around 94 Mt was produced by thermal methane reforming, a process that emits GHGs, and less than 1 Mt was low-carbon hydrogen, the majority of which came from methane reforming with carbon capture and utilization. But the situation is expected to change in the near future. Low-carbon hydrogen production in 2030 is projected to reach 38 Mt.
China is leading the nascent low-carbon hydrogen market with 30% of all production, followed by the USA, the Middle East, India, and Russia, according to the IEA’s annual report. Brazil’s participation is small. “It is estimated that in 2022, the country produced 509,000 tons [t] of hydrogen from natural gas and only 29,000 tons by electrolysis,” says Gustavo Pires da Ponte, and engineer from EPE.
This volume is expected to grow in the coming years as a result of various ongoing initiatives and projects. In June, the São Paulo government launched a program designed to encourage the decarbonization of production chains in the state. Part of the initiative is the São Paulo Green Roadmap, which includes a low-carbon hydrogen program created to stimulate the state’s potential for different hydrogen production pathways.
“We want to develop the value chain for the entire low-carbon hydrogen industry, which includes equipment, components, services, and training, without preselecting one technological pathway or another,” explains Marisa Maia de Barros, undersecretary of energy and mining at the São Paulo State Department for the Environment, Infrastructure, and Logistics. “The goal is to stimulate the production of low-carbon hydrogen, leveraging São Paulo’s experience in the energy sector.”

Léo Ramos Chaves / Pesquisa Fapesp
Sugarcane: ethanol produced from the plant can be converted into hydrogenLéo Ramos Chaves / Pesquisa FapespThe state of São Paulo, according to Barros, has the potential to produce hydrogen from several sources, including ethanol and biomethane, a gaseous biofuel generated by processing waste from the sugar-energy sector. “Biomethane and natural gas are exactly the same molecule [CH4]. The difference is that the former is of renewable origin and the latter has fossil origins. The same technology we have long used to produce hydrogen from natural gas can also use biomethane,” says the undersecretary. “The challenge is to scale biomethane production.”
The most important work on producing hydrogen from products or byproducts of the sugar-energy sector, such as ethanol and vinasse, is being done by the Center for Research and Innovation in Greenhouse Gases (RCGI), an Engineering Research Center (CPE) founded jointly by FAPESP and Shell, which has received investments of R$465 million since its creation in 2015, with R$45 million from FAPESP. The center is working on three fronts—the most advanced research involves the steam reforming of ethanol.
In this method, the fuel is subjected to specific temperatures and pressures and thus reacts with water in a chemical reactor, resulting in hydrogen (see infographic below). Biogenic carbon of non-fossil origin, from the sugarcane, is emitted during the process. A demonstration unit is being built at the University of São Paulo (USP).
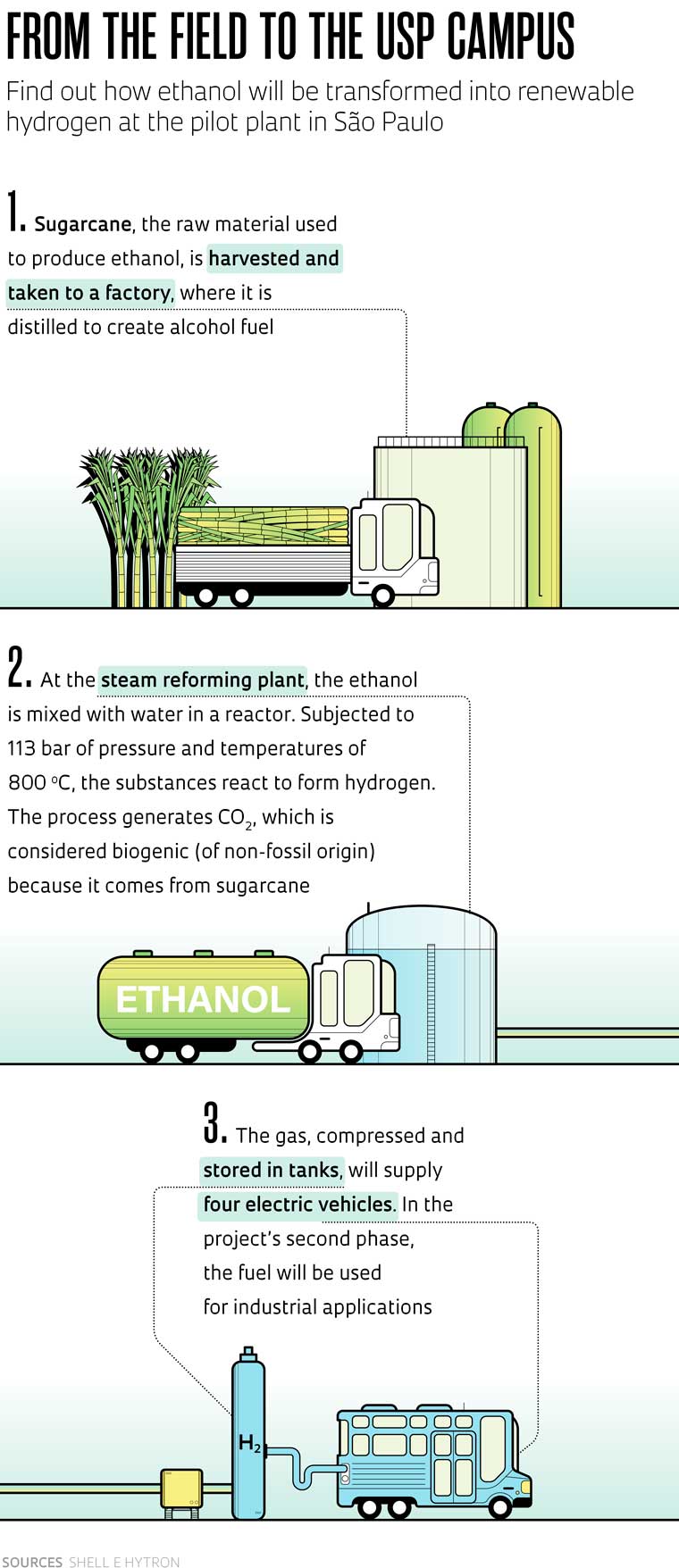
The project, worth R$50 million and funded by Shell, is partnered with Brazilian energy companies Hytron and Raízen, Japanese automaker Toyota, the National Service for Industrial Training (SENAI), and USP, via the RCGI. The pilot plant, scheduled to begin operating in the second half of 2024, will have 425 square meters of floor space and the capacity to generate 4.5 kg of hydrogen per hour.
“It will be the world’s first experimental renewable hydrogen fueling station using ethanol,” says engineer and physicist Julio Meneghini, scientific director of RCGI and a professor at USP’s Polytechnic School. The fuel will be used in three buses and a car, all electric and equipped with a device called a fuel cell, which generates electricity from hydrogen without emitting any GHGs.
“If the ethanol has a negative CO2 footprint in its production process, meaning it eliminates more carbon than it emits, then the hydrogen generated will have a negative footprint,” says Meneghini. Ethanol can be carbon negative if nitrogen fertilizers are not used by the sugarcane farms and no fossil fuels are used by the agricultural machinery and trucks transporting the input. The carbon emitted in the sugarcane fermentation process also has to be captured and stored.

Rubens Cavallari / Folhapress
Toyota car that runs on hydrogen generated from alcohol fuelRubens Cavallari / FolhapressThiago Lopes, project coordinator at RCGI, highlights an advantage of hydrogen generated from thermal ethanol reforming: transport logistics. Transporting the hydrogen produced by electrolysis is complex and expensive, he explains, because the gas has to be compressed in high-pressure cylinders or liquefied in cryogenic tanks (stored at extremely low temperatures), which makes it costly to ship the fuel from the production site to its destination. “This problem does not exist for hydrogen made from ethanol. Ethanol already has an established transport chain. It is much easier to transport it in liquid form than to compress or liquefy the hydrogen,” says Lopes.
The project’s ethanol reformer was developed by Hytron. “We designed this new technology with funding from FAPESP and we have now reached a precommercial stage. By testing the equipment at USP, we will be able to advance the maturity of the technology and reach a commercial level,” highlights Daniel Lopes, commercial director at Hytron.
The RCGI is also investigating the transformation of ethanol into hydrogen through electrochemical reforming. “Electricity is used to break down the ethanol molecule, generating hydrogen in a process similar to water electrolysis,” explains the project coordinator, chemical engineer Hamilton Varela, director of USP’s São Carlos Chemistry Institute (IQSC).
According to Edson Antonio Ticianelli, a chemist who is participating in the study and is part of FAPESP’s Bioenergy Research program (Bioen), one of the goals was to replace the electrolyzer’s anodic reaction, which in a conventional system is the oxidation reaction of water, with the oxidation of ethanol, thus improving the energy efficiency of the process. The researchers are investigating materials that could be used as catalysts for breaking down the ethanol molecule. Once this challenge has been overcome, the next step will be to develop an electrochemical reformer for ethanol.
The third line of research uses vinasse as a raw material. Every liter of ethanol produced is accompanied by around 12 liters of vinasse, a byproduct that is 95% water. Vinasse has a high potential for contaminating groundwater and emitting GHGs. To minimize these effects, it is often reused as biofertilizer for sugarcane crops. In the RCGI project, vinasse is concentrated in an electrochemical reactor to reduce the amount of water and generate sustainable hydrogen and oxygen. The group has already filed a patent for the process.
In addition to the three pathways investigated by RCGI, other studies in Brazil are looking into the direct use of ethanol in solid oxide fuel cells (SOFCs). In this approach, the ethanol molecule is broken down to generate hydrogen in the engine of the vehicle, rather than at an independent fueling station, like in the USP project. Research in this field is being carried out at the Institute for Energy and Nuclear Research (IPEN), with funding from FAPESP (see Pesquisa FAPESP issue nº 308).

Electrolytic hydrogen
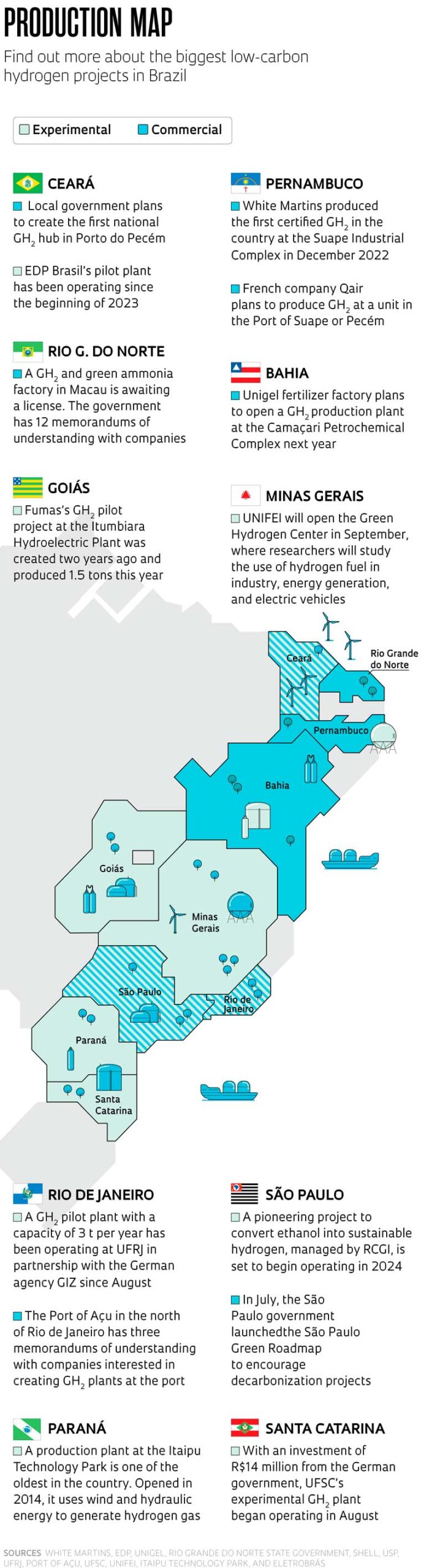
In addition to research focused on converting biomass and biofuels into low-carbon hydrogen, other studies in Brazil are attempting to generate green hydrogen (GH2), the production of which must emit zero or practically zero CO2 (see infographic on page 19). There are both commercial and experimental plants, most in their early stages. At the Suape Industrial Complex in Pernambuco, industrial gas manufacturer White Martins began production in 2022 of the first GH2 certified in Brazil and in South America. Powered by solar energy, the plant can produce 156 tons of the gas per year, to be used by the food industry in the region.
The Unigel nitrogen fertilizer factory in Bahia intends to start producing green hydrogen on an industrial scale using wind energy at the Camaçari Petrochemical Complex in 2024. At a cost of R$120 million, the factory will be able to produce 10,000 tons of the gas per year. The plan is to quadruple the production capacity within two years.
Another project already operating in the country is based at the Pecém Thermoelectric Complex in Ceará, where the energy company EDP Brasil produces GH2 at a pilot plant with the capacity to produce 197 t per year from solar energy. The Ceará government also intends to establish the first national GH2 hub in Porto do Pecém.

EDP
EDP pilot plant at the Pecém Thermoelectric Complex in CearáEDP“Thirty-three memorandums of understanding have already been signed with Brazilian and foreign companies, three of which have advanced to the precontract phase,” says Joaquim Rolim, executive secretary for industry at the Ceará State Department for Economic Development. “Commercial production is set to begin in 2026 or 2027 and we plan to generate around 1 Mt of green hydrogen per year from 2030 onwards,” he explains.
“Ceará, like the entire Northeast, has great potential for generating wind and solar energy, the main input for green hydrogen production. The state’s location, closer to ports in Europe and North America, favors the export of renewable fuel,” says chemical engineer Diana Azevedo, vice dean of the Federal University of Ceará (UFC) and director of the university’s Technology Center.
Azevedo, who is also a professor at UFC’s Department of Chemical Engineering, is leading research into the green hydrogen production chain, specifically storage and transportation of the fuel. “Transporting hydrogen from where it is produced to where it is consumed is challenging, as it is an extremely light gas that requires a lot of energy to be stored, either in gaseous or liquefied form,” she explains. One way to overcome this problem is to transform hydrogen into liquid compounds, such as ammonia, or incorporate it into solids, such as magnesium hydride, and then recover it at the destination. “Our research is looking into the creation of composites with magnesium, iron, and carbonaceous supports for chemical storage of hydrogen,” says Azevedo.
Research into the production of low-carbon hydrogen is also being carried out at other Brazilian universities and research centers, including UFSC and the Federal University of Rio de Janeiro (UFRJ), which recently opened pilot GH2 plants. Both initiatives are funded by the German Agency for International Cooperation (GIZ). “Our project has the capacity to produce 3 t of hydrogen per year via electrolysis powered by photovoltaic energy,” says Andrea Santos, coordinator of the project and a professor at UFRJ’s Alberto Luiz Coimbra Institute for Engineering Research and Graduate Studies (COPPE).
“We are going to test the fuel in hybrid electric bicycles powered by hydrogen and fuel cells, in industrial processes, and in solid oxide fuel cells. We will also investigate new catalysts for producing biofuels and sustainable aviation fuels [SAFs], which can be manufactured from GH2,” says Santos.
In September, together with colleagues from UFRJ, she published a review of hydrogen production in the country from a technical and economic perspective. According to the article, published in the journal Energies, “electrolysis is the most studied process in the literature, as it reduces greenhouse gas emissions and presents other advantages, such as maturity, energy efficiency, flexibility, and energy storage potential.”

Fernando Souza / Giz Brasil
Hydrogen-powered hybrid electric bicycles on the UFRJ campusFernando Souza / Giz BrasilObstacles in the road
Despite Brazil’s potential in low-carbon hydrogen production, many challenges still need to be overcome before the country can scale up production, starting with the final price. According to the IEA, the cost of producing hydrogen from fossil fuels in 2022 was between US$1.5 and US$6.1 per kg, while low-carbon hydrogen from fossil fuels cost between US$1.8 and US$7.6 per kg. The cost of green hydrogen ranged from US$3.8 to US$12 per kg.
There are several variables involved in lowering the cost of GH2, including the availability and price of renewable electricity used to convert water into the gas. “The value of electricity is a crucial element in the cost of GH2,” warns Caroline Chantre, an economist from UFRJ’s Electricity Sector Study Group (GESEL). Chantre was coauthor of a 2022 article in the journal Sustainable Production and Consumption that analyzed the perception of economic stakeholders in Brazil’s developing hydrogen market. “Our study showed that 43% of stakeholders saw the medium-term, of between 6 and 10 years, as a best estimate for GH2 becoming a reality in the country,” she says.
Another obstacle to increasing the production scale of GH2, according to an article published in Nature Energy in 2022, is the scale of electrolyzer production and construction of plants adopting this approach. “Even if electrolysis capacity grows as quickly as wind and solar power, GH2 supply will remain scarce in the short term and uncertain in the long term,” the authors point out.
Making electrolyzers more efficient is part of the solution to the problem. One field of research is the development of low-cost, easy-to-produce, energy-efficient catalysts to increase the speed of chemical reactions in electrolysis. This is the focus of the work being done by chemist Lucia Helena Mascaro, a professor at the Federal University of São Carlos (UFSCar) and a researcher at the Center for Innovation in New Energy (CINE), a CPE funded by FAPESP.
The group uses composite materials made of nickel, molybdenum, and copper; nickel and phosphorus; nickel, cobalt, and phosphorus; and molybdenum and sulfur. “These alloys have high stability and low potential for the water reduction reaction, resulting in good energy efficiency,” says Mascaro. Based on the results of the research, published in the Journal of the Electrochemical Society, ACS Applied Materials & Interfaces, and other scientific journals, the team set out to assemble and test electrolyzer prototypes with new catalysts on a larger scale and closer to the real system.
Experts also highlight the need to align the perspectives of producers and consumers, creating a demand for hydrogen. “This issue will begin to be resolved when large-scale demand arises, as is being seen in Europe, stimulating investments in Brazil,” says Ruther, from UFSC.
The development of a regulatory framework and the establishment of incentivizing public policies are also essential to stimulate progress in the various incipient low-carbon hydrogen projects across the country. “Regulation is a key element in ensuring stability and security for investments,” says Chantre. “Although Brazil has significant competitive advantages, we still need to move forward in terms of public policies aligned with a long-term decarbonization strategy.”
Projects
1. Cine – Advanced Energy Storage Division (nº 17/11958-1); Grant Mechanism Engineering Research Centers (CPEs); Principal Investigator Rubens Maciel Filho (UNICAMP); Investment R$8,646,512.62.
2. Research Center for Innovation in Greenhouse Gases (RCGI) (nº 20/15230-5), Grant Mechanism Engineering Research Centers (CPEs); Principal Investigator Julio Romano Meneghini (USP); Investment R$17,261,689.15.
3. Integrated unit for hydrogen production from autothermal reforming of ethanol (nº 14/50183-7); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator Daniel Lopes (Hytron); Investment R$618,861.42.
4. Development and integration of an integrated ethanol reforming unit for hydrogen production (nº 05/50908-2); Grant Mechanism Innovative Research in Small Businesses (PIPE); Principal Investigator João Carlos Camargo (Hytron); Investment R$483,555.25.
5. Effect of electrolyte properties on electro-oxidation of alcohols on platinum (nº 22/08723-0); Grant Mechanism Thematic Project; Principal Investigator Hamilton Varela (USP); Investment R$226,823.31.
6. Electrocatalysis VI: Fundamental and applied aspects of emerging and classical problems in electrochemical energy conversion (nº 19/22183-6); Grant Mechanism Thematic Project; Principal Investigator Edson Antonio Ticianelli (USP); Investment R$6,415,517.84.
7. Research Division 1: Dense energy carriers (nº 17/11986-5); Grant Mechanism Energy Research Centers (CPEs); Principal Investigator Ana Flávia Nogueira (UNICAMP); Investment R$10,273,057.80.
Scientific articles
CHANTER, C. et al. Hydrogen economy development in Brazil: An analysis of stakeholders’ perception. Sustainable Production and Consumption. Vol. 34, pp. 26–41. Nov. 2022.
MEDINA, M. et al. The substrate morphology effect for sulfur-rich amorphous molybdenum sulfide for electrochemical hydrogen evolution reaction. Journal of the Electrochemical Society. Vol. 169, no. 2. Feb. 2022.
ODENWELLER, A. et al. Probabilistic feasibility space of scaling up green hydrogen supply. Nature Energy. Vol. 7, pp. 854–65. Sept. 2022.
SANTOS, H. et al. NiMo-NiCu inexpensive composite with high activity for hydrogen evolution reaction. ACS Applied Materials & Interfaces. Vol. 12, pp. 17492–501. Mar. 2020.
SANTOS, H. et al. Effect of copper addition on cobalt-molybdenum electrodeposited coatings for the hydrogen evolution reaction in alkaline medium. International Journal of Hydrogen Energy. Vol. 45, pp. 33586–97. Nov. 2020.
CAMARGO, J. C. et al. Termodinâmica do uso do hidrogênio obtido via reforma etanol para aplicações em sistemas com células a combustível. Annals of the IX Brazilian Congress on Thermal Engineering and Sciences. Caxambu (MG), 2002.


