 PEDRO HAMDANTwo studies published in the journal Science in early July renewed the hope that one day, even if it is in the distant future, a safe and effective HIV vaccine will be produced. The HIV virus, over the last three decades, infected 60 million people worldwide and killed 27 million, a number of victims perhaps only less that of the 1918 flu epidemic and of World War II. In one of the studies, researchers from two American universities and from the National Institutes of Health (NIH) of the United States, the world’s largest medical research center, isolated two highly powerful antibodies from the blood of an Aids patient. Each of the antibodies (VRC01 and VRC02) neutralized 91% of the 190 most common HIV varieties, a far better performance than that of the most efficient antibodies found previously, which only blocked 40% of the strains. In another study, a team that included the Brazilian immunologist Michel Nussenzweig, from Rockefeller University, analyzed the structure and molecular characteristics of VRC01 and identified the area of the virus with which this antibody bonds, which makes it impossible for HIV to infect human cells.
PEDRO HAMDANTwo studies published in the journal Science in early July renewed the hope that one day, even if it is in the distant future, a safe and effective HIV vaccine will be produced. The HIV virus, over the last three decades, infected 60 million people worldwide and killed 27 million, a number of victims perhaps only less that of the 1918 flu epidemic and of World War II. In one of the studies, researchers from two American universities and from the National Institutes of Health (NIH) of the United States, the world’s largest medical research center, isolated two highly powerful antibodies from the blood of an Aids patient. Each of the antibodies (VRC01 and VRC02) neutralized 91% of the 190 most common HIV varieties, a far better performance than that of the most efficient antibodies found previously, which only blocked 40% of the strains. In another study, a team that included the Brazilian immunologist Michel Nussenzweig, from Rockefeller University, analyzed the structure and molecular characteristics of VRC01 and identified the area of the virus with which this antibody bonds, which makes it impossible for HIV to infect human cells.
“These results are most encouraging and unveil a new path for the development of an HIV vaccine,” stated the immunologist Anthony Fauci, one of the world’s most highly respected Aids researchers and the director of the National Institute of Allergies and Infectious Diseases, one of the 27 centers that are part of the NIH, in an e-mail interview granted to Pesquisa FAPESP.
These studies, rather than representing the success of an elite scientific group, reiterate the correctness of a new era in the quest for vaccines, an era currently referred to as “the third wave.” It began two or three years ago and aims to correct the problems found in the preceding stages, in which dozens of formulations of potential HIV vaccines were studied. Of the 30 that underwent some human trials, only one produced any degree of protection, albeit very low. In the light of these thwarted attempts and of a global investment of almost US$1 billion a year in recent years, the international scientific community and organized segments of society got together, reviewed the vaccine development targets and decided to invest more effort, time and money in more innovative and potentially more efficient technologies. It was a change in direction that made room for the participation, even if incipient, of Brazilian teams in the race toward a vaccine.
“There has been substantial progress in this area in the last two years and Brazil mustn’t lag behind,” says Cristina Possas, who is in charge of the Technological Development and Research Unit of the STD, Aids and Viral Hepatitis Department of the Ministry of Health. Along with the Federal University of Rio de Janeiro and Iavi (the International Aids Vaccine Initiative), Cristina’s group is conducting a survey of the domestic research and technological development capabilities regarding HIV vaccine production. Preliminary data from this study, to be presented in October at the Brazilian Academy of Sciences, indicate that the Brazilian investment is modest, coming largely from the Ministry of Health. Since 1999, this ministry has invested R$7 million in 31 studies of compounds with the potential of becoming an Aids vaccine, or 95% of what was spent on this field in Brazil, whereas South Africa invested US$200 million. “We must expand partnership agreeements with other finance agencies in the country,” states Cristina.
At the International Aids Conferences held in July, Cristina recalls, it became clear that it is a mistake to assume that medication alone can keep Aids in check. The World Health Organization has estimated that only 40% of Aids carriers have access to medication. According to estimates, out of every two people who start treatment, another three get the virus. A pioneer in the distribution of antiretroviral drugs free of charge, Brazil has been investing increasing amounts of money, to a total of R$1 billion in 2009, for the treatment of Aids. “One of the Ministry of Health’s largest expenditures is anti-HIV medication,” says the immunologist Ernesto Torres Marques, a member of the ministry’s National Advisory and Technical Committee on HIV Vaccines.
Researchers and members of the organized segments of society at large generally agree that, in order to contain the Aids pandemic, it will be necessary to resort to all possible means, including sex education campaigns and guidance about safe sex, plus a vaccine, of course. “If Brazil maintains a posture of waiting for others to solve the problem, in the future we’ll have to pay for whatever they develop,” says Marques, a researcher at both the University of Pittsburgh and the Oswaldo Cruz Foundation. He has created a technology that tries to make it easier for possible vaccine components to enter the cells and thus to heighten the body’s immunological response.
Ever since HIV was identified in 1983 as the cause of Aids, the syndrome that annihilates the human defense system and makes it susceptible to various infections, researchers around the world have been searching for antibodies as powerful as those described in Science, but with no success. Most of the antibodies adhered to specific strains of the virus without neutralizing them. The discovery presented in July restores researchers enthusiasm after years of feeble results, because it proves an old hypothesis. If the organism of an HIV carrier was able to produce such effective antibodies naturally, it should be possible, in theory at least, to stimulate other people to make them as well, by giving them the vaccine.
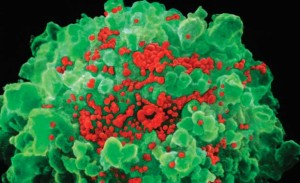
NIBSC / SCIENCE PHOTO LIBRARY / SPL DC / LATINSTOCKRed tide: infected T lymphocyte (green) releases new copies of HIV NIBSC / SCIENCE PHOTO LIBRARY / SPL DC / LATINSTOCK
However, it will be neither fast nor easy to get to such a vaccine. First, one must discover how to reproduce artificially the proteins of the surface of the virus that, in contact with defense cells, lead to the production of these powerful antibodies, find the most efficient way of introducing them into the body and assess whether they do indeed work. Only then, once the industrial production stages and animal and human trials required by governmental health institutions to authorize sales have also been completed, will the sought-after vaccine, capable of keeping healthy people from getting HIV, be achieved. For the time being, the closest thing to a vaccine is the combination of two formulations (Aidsvax and Alvac-HIV) that did not work separately. The combination was tested from 2003 to 2006 in Thailand in a clinical trial involving 16.4 thousand people – half of them treated with the dual formulation and the other half with a placebo. The effectiveness of the combination was considered low: the number of people infected was 31% lower in the group given Aidsvax plus Alvac than in the control group.
Despite this disappointment, these results, presented in 2009 in the New England Journal of Medicine, have a positive side. For the first time ever, scientists verified in humans something that had previously only been found in monkeys: it was possible to use a vaccine to stimulate the production of antibodies against HIV.
Two years earlier, clinical trials using a possible formulation for a vaccine, produced by the pharmaceutical company Merck, had to be suspended early because it provided no protection. This compound worked differently from the combination used in Thailand. Instead of stimulating the production of antibodies (a humoral response), it activated cell defense: it recruited a chain of cells to identify particles of invading microorganisms and to eliminate infected cells.
The Merck vaccine resorted to a modified version of a virus that attacks the respiratory system, the human adenovirus type 5, which cannot reproduce, in order to insert artificial genes of three HIV proteins in human cells. Once inside the cells, in particular of a group called dendritic cells, the HIV genes start to produce copies of these proteins, which are then stuck on the cell membrane, where they work like a neon sign advertising the presence of the invader. Other defense cells, the T CD8 lymphocytes, identify these warnings and release toxic proteins that kill the infected cells. The idea was a good one and had it worked, it might have led to a different type of vaccine, one that induced cell immunity.
 Pedro HamdanHowever, Merck’s compound did not have the expected effect. Initially, researchers thought that it might even increase the risk of HIV infection among people who had already had contact with the adenovirus. This possibility, later refuted, was due to the fact that their antibodies kept the vaccine’s adenovirus from entering the cells and therefore from unleashing a cellular response. “In Brazil, approximately 70% of the population has antibodies against this virus, indicating that the Merck vaccine might not work here,” states the immunologist Aguinaldo Pinto, from the Federal University of Santa Catarina (UFSC). Along with his team, he is trying to solve this problem by replacing the human adenovirus by a strain only found in chimpanzees. In trials using mice, the formulation, applied to the vaginal, nasal and oral mucous membranes, stimulated the proliferation of defense cells in other mucous membranes of the body. Nevertheless, the issue is not that simple. “After the first dose, the body may develop antibodies, making it necessary to use another type of virus for the booster dose,” he explains.
Pedro HamdanHowever, Merck’s compound did not have the expected effect. Initially, researchers thought that it might even increase the risk of HIV infection among people who had already had contact with the adenovirus. This possibility, later refuted, was due to the fact that their antibodies kept the vaccine’s adenovirus from entering the cells and therefore from unleashing a cellular response. “In Brazil, approximately 70% of the population has antibodies against this virus, indicating that the Merck vaccine might not work here,” states the immunologist Aguinaldo Pinto, from the Federal University of Santa Catarina (UFSC). Along with his team, he is trying to solve this problem by replacing the human adenovirus by a strain only found in chimpanzees. In trials using mice, the formulation, applied to the vaginal, nasal and oral mucous membranes, stimulated the proliferation of defense cells in other mucous membranes of the body. Nevertheless, the issue is not that simple. “After the first dose, the body may develop antibodies, making it necessary to use another type of virus for the booster dose,” he explains.
Aware of these results, the immunologist Edecio Cunha Neto, from the Heart Institute and the Medical School of the University of São Paulo (USP), decided to invest in the development and testing of a compound, the production of which was based on the new premises, possibly one of the first Brazilian candidates for an HIV vaccine.
Around 2002, immediately following his return from spending some time at Harvard, Cunha Neto joined the team of the immunologist Jorge Kalil. Together, they began analyzing the defense system of a special group of HIV-infected people who had maintained HIV under control for longer and who took more time to become ill. In their blood, the number of T lymphocytes of the CD4 type remained higher than normal. Responsible for activating the T lymphocytes that produce toxins (CD8) and the producers of antibodies (B lymphocytes), the CD4 cells are the chief target of HIV, which makes use of the cell machinery to reproduce. However, the researchers still had to figure out what was the special feature of the CD4 lymphocytes of these people.
One possibility they came up with was that the CD4 lymphocytes might recognize the virus and help other lymphocytes to fight it. To test this idea, they isolated small segments of HIV proteins and started a sort of molecular fishing effort, to see which of these segments was more easily recognized by these patients’ lymphocytes. They selected 18 fragments of proteins (peptides) from areas of the virus that remain unchanged in most of the strains and had been identified by the CD4 more often, and then they recreated them in a laboratory.
 A test conducted by immunologist Simone Fonseca, from Cunha Neto’s team, using blood samples from 32 persons with HIV, showed that the defense cells of virtually all of them recognized at least one of the peptides. In 40% of the cases, more than five peptides were identified, according to a study published in 2006 in Aids.
A test conducted by immunologist Simone Fonseca, from Cunha Neto’s team, using blood samples from 32 persons with HIV, showed that the defense cells of virtually all of them recognized at least one of the peptides. In 40% of the cases, more than five peptides were identified, according to a study published in 2006 in Aids.
In another experiment, described in an article published in June 2010 in PlosOne, Susan Ribeiro and Daniela Rosa administered the peptides to mice that had been genetically modified to produce molecules of the human immune system. The results were even more encouraging: 16 of the 18 peptides were recognized and activated both the CD4 and the CD8 lymphocytes. “If everything goes well, these peptides may work like a booster for HIV vaccines, such as the Merck one, which already has complementary principles,” Cunha Neto commented.
Before these formulations can be used on humans, a long journey of trials lies ahead, and it is fraught with obstacles, such as the lack of suitable infrastructure in Brazil for the more advanced trials. Experiments on monkeys, whose organism is closer to that of humans, cost at least US$500 thousand. A further US$7 million would be required to produce a suitable formulation for human use. “Our hope,” says Cunha Neto, “is that the results of the trials surpass expectations, making it easier to get funding for the subsequent stages, which are increasingly expensive.”
Also at USP, the group of the immunologist Alberto da Silva Duarte, chairman of the ministry’s vaccine committee, is starting a second stage of human trials with another vaccination strategy, first evaluated in Recife by Luis Arraes. Called a therapeutic vaccine, it is prepared with the defense cells of the blood of the infected person. These cells are cultivated with inactive copies of the virus before they are reintroduced into the body. It is hoped that this type of vaccine may help the immune system of HIV patients to keep the virus under control for longer, delaying the need for antiretroviral medication. “A therapeutic vaccine, besides complementing treatment, creates an opportunity to find out which type of immune response generates protection against HIV,” states Duarte.
Both in Brazil and abroad, the cost of these experiments limit the development of potential vaccines. The formulation created by Ernesto Marques is at an advanced trial stage. Experiments with 40 rhesus monkeys have shown that this formulation speeds up lymphocyte identification of HIV and increases the level of these defense cells in the blood. Studies comparing this formulation with another, using a monkey virus, showed the formulation’s efficacy as a therapeutic strategy. Marques, who is collaborating with Alberto Duarte’s group, is now looking for US$1.5 million in funding to start human trials. In 2008, when the most recent version of the Brazilian HIV Vaccine Plan was launched, the Health Minister, José Temporão, promised to commit R$25 million to the development of vaccines. To date, however, the funds have not been released, according to Cristina Possas.
The projects
1. Protective immune response against infection by HIV: development of immunogens against HIV-1 and identification of targets for immunological intervention on slowly progressing patients (nº 2006/57179-9); Type Regular Research Awards; Coordinator Edecio Cunha Neto – FMUSP; Investment R$ 302,296.32.
2. Use in vaccines, immunogens and evaluation trials of the cellular immune response to HIV-1 of epitopes of unprecedented T CD4+ lymphocytes from conserved regions of HIV-1 subtype B (nº 2004/15331-3); Type Program of Support for Intellectual Property (Papi); Coordinator Edecio Cunha Neto – FMUSP; Investment R$ 60,030.81.
3. Identification of viral, genetic and immunologic factors associated with the phenotype of non-progression of the HIV disease (nº 2001/00729-3); Type Regular Research Awards; Coordinator Edecio Cunha Neto – FMUSP; Investment R$ 324,895.73.
Scientific article
RIBEIRO, S. P. et al. A vaccine encoding conserved promiscuous HIV CD4 epitopes induces broad T cell responses in mice transgenic to multiple common HLA class II molecules. PlosOne. v. 5(6). 11 June 2010.