
Richard Janissen/Delft University of TechnologyEPS filaments projecting from a biofilm, in colorized images obtained with a fluorescence microscopeRichard Janissen/Delft University of Technology
“What are these elongated cells?” asked physicist Mônica Cotta, pointing to images of the bacterium Xylella fastidiosa produced by the powerful microscopes at the National Institute of Photonics Applied to Cell Biology (Infabic) at the University of Campinas (Unicamp). The question evoked a nearly forgotten memory from previous studies by biologist Alessandra de Souza, a researcher at the Sylvio Moreira Citriculture Center of the Campinas Institute of Agronomy (IAC). Under stressful conditions, many bacteria stop producing the protein that cleaves them in two after replication. This is called filamentation and it appears to be a key element in forming the biofilm that makes Xylella so feared: the bacteria assemble into clusters that occupy and obstruct a plant’s xylem (the vessels that transport water and other substances from the root to other parts of the plant), wreaking serious havoc on plant development and fruit production. The group described the formation of this biofilm in an article published in April 2015 in Scientific Reports, and they continue to search for ways to defeat this long-standing scourge of Brazilian citrus, American grapes and, most recently, Italian olives. Their best chance of success may lie, at least in part, in an interdisciplinary partnership. According to physicist and Infabic vice-coordinator Carlos Lenz Cesar, the institute’s biggest strength is that its researchers come from various fields of specialization. “Each is expected to learn more about their field and contribute to the whole through collaboration,” he says.
When Cotta first started analyzing Xylella through her fluorescence microscope, she was unable to obtain good images because the light emitted by the bacteria – which the equipment needs in order to make an image – was too weak. Things improved when Souza suggested injecting the bacteria with green fluorescent protein (GFP), whose much brighter shine has made it the most successful marker for this type of biological study. Even so, the equipment does not permit the study of live bacteria, and there was still no explanation for the little green circles that also showed up under an atomic force microscope, used by the physics group at the laboratory. After years of scrutinizing materials through a microscope, it was Souza’s trained eye that caught the fundamental clue. “Bacteria can’t show this type of symmetry unless they are ‘standing on end’ or, in other words, vertical,” she imagined (see image). What she was seeing was probably a bird’s eye view of bacterial extremities. So she turned to other types of microscopy.
The confocal microscope at Infabic, which can generate three-dimensional images, was a promising option. But the live bacteria still appeared blurred. The solution emerged with the arrival of a confocal microscope with spinning disk technology. This type of microscope is equipped with a disk covered in tiny pinholes, each fitted with a lens. As the disk spins at high speed, the sample is scanned with laser beams that pass through these lenses. “It allowed us to produce 3-D images in a few seconds, instead of several minutes like a traditional confocal microscope,” says the physicist. Her group – specifically, German biologist Richard Janissen, then a post-doctoral researcher at Infabic and now working at the Delft University of Technology, in the Netherlands, and Colombian physicist and doctoral student Duber Murillo – started examining Xylella cell-culture dishes throughout the entire life cycle of the organism, discovering an important part of the bacteria’s behavior. Cotta’s initial interpretation had been correct. “The bacteria ‘stand on end’ and rotate around the end that is attached to the surface,” she explains, demonstrating a swaying movement resembling that of an inflatable bobo doll. The images were blurred because of this movement. “With this microscope, we can produce 100 frames per second, in three dimensions,” says Lenz. “We catch the bacteria mid-movement, like photographing a helicopter in flight and getting a picture with the rotary blades seeming completely still.”
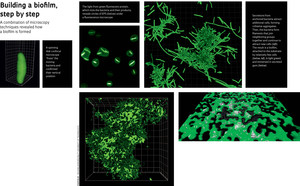 Fluorescence microscopy and the chemical signatures detected with the confocal Raman microscope at the University of São Paulo (USP) showed that the bacteria secrete materials that accumulate at one of their extremities, early during the colonization process. These secretions, generically known as extracellular polymeric substances (EPS), exhibit different properties according to the development stage of the biofilm. At an initial moment, soluble EPS anchors the bacteria to the substrate (in this case, the glass dish), but in a reversible manner. After a sample is washed at this stage, the microscope will reveal volcano-shaped structures where the bacteria used to be attached. Then, the cells start producing capsular EPS, which makes the adhesion irreversible and attracts new bacteria, leading to the formation of cell aggregates. The bacteria become embedded in an EPS “gum”, and so a Xylella biofilm is born.
Fluorescence microscopy and the chemical signatures detected with the confocal Raman microscope at the University of São Paulo (USP) showed that the bacteria secrete materials that accumulate at one of their extremities, early during the colonization process. These secretions, generically known as extracellular polymeric substances (EPS), exhibit different properties according to the development stage of the biofilm. At an initial moment, soluble EPS anchors the bacteria to the substrate (in this case, the glass dish), but in a reversible manner. After a sample is washed at this stage, the microscope will reveal volcano-shaped structures where the bacteria used to be attached. Then, the cells start producing capsular EPS, which makes the adhesion irreversible and attracts new bacteria, leading to the formation of cell aggregates. The bacteria become embedded in an EPS “gum”, and so a Xylella biofilm is born.
It was in sequential images of biofilm formation that Janissen first saw stringy bacteria – later explained as filamentation – among clusters of neighboring cells. “The aggregates probably produce a chemical signal that tells some bacteria along the border to start replicating without dividing,” Cotta explains. The result is elongated bacteria that secrete EPS, attract more bacteria, and accelerate the formation of a cohesive community. According to the physicist, this is the first description of filamentation in bacterial biofilms. She discovered what appears to be the same phenomenon in the supplementary materials of a paper about the cholera bacterium, published by an American research group in the journal Science in 2012. “I saw a video in which the filaments suddenly appeared, but the authors do not mention anything about it,” says Cotta. The information being visible is not enough: someone has to actually see it.
Another aspect of the bacterium’s life cycle was revealed thanks to confocal microscopy, which allows researchers to rotate a sample virtually and analyze it from below, where the biofilm anchors itself to the Petri dish. The biofilm is attached by only a few cells, those initial bacteria that adhered to the substrate by one of their extremities. This structure can facilitate the transmission of Xylella disease by its insect vector. “It becomes easy for the leafhopper to tear away a piece of the biofilm as it sucks the sap from the infected plant,” says Cotta, although this is still only a speculation. Whether this truly happens remains to be seen; for now, this exemplifies the kind of clue that physical knowledge can turn up for biologists. “What we have does not contradict what Xylella needs in vivo.”
In practice
Murillo is now trying to determine what components are needed for the bacterial cells to adhere to a culture medium. In this exploratory process, the physicist has been removing one component at a time and then adding it back again, observing the reaction of Xylella all the while. Cotta jokingly calls this part of the job “biologist work”. The idea is to design an adhesion model that will enable researchers to address the problem using a more experimental approach, trying to prevent the biofilm from forming, for example.

Richard Janissen/Delft University of Technology
Perpendicular to the substrate, bacteria secrete adhesive substance (EPS)Richard Janissen/Delft University of TechnologyOne goal is to understand how N-acetylcysteine (NAC) prevents Xylella fastidiosa from causing severe damage to plants, as shown by Souza’s group in 2013 (see Pesquisa FAPESP Issue No. 214). For now, Cotta suspects that NAC, an ingredient in cough syrups, acts on soluble EPS and prevents adhesion from becoming irreversible. At this initial moment of biofilm formation, Indian physicist Prasana Sahoo, who is a post-doctoral intern at Infabic, added NAC to the sample he was observing under the confocal microscope. He saw bacteria detach themselves from the substrate.
This was a collateral observation in Sahoo’s research. He places bacteria on a surface covered in spiky nanowires, resembling a miniature bed of nails. EPS molecules form a web that bends the wires, which in turn record the amount of force exerted by the biofilm.
According to Cotta, these advances were made possible by combining different microscopy equipment and techniques to help fit the puzzle pieces together, little by little. Nothing new in the field of material physics. “We simply transferred the same reasoning to microbiology,” she says.
Severely damaging to Brazil’s orange industry, Xylella fastidiosa recently arrived in the south of Italy, where it has been terrorizing the region of Apulia. The centuries- (or even millenia-old) olive trees in the area are a national heritage. In 2013, plant virologist Donato Boscia, from the National Research Council in the city of Bari, saw desiccated olive trees in a grove and set out to find the cause. After consulting with colleagues in Italy and other countries, he ran into Xylella fastidiosa. In early 2014, a researcher in his group traveled to Cordeirópolis, a city in inland São Paulo State, to learn how to isolate the bacteria, together with agronomist Helvécio Della Coletta-Filho, head of the phytopathology clinic at the Citriculture Center. Upon returning to Italy, she quickly accomplished this. “I’ve never seen a group advance their research so far in so little time,” says Souza.
A genetic comparison of Xylella samples from different parts of the world indicates that the bacteria in Italian olive groves likely arrived in Europe by hitchhiking on ornamental plants (oleander) from Costa Rica. The cultural bond between Italians and olives makes the bacterial invasion a social problem, in addition to an economic one. “Some people are even blaming the researchers who discovered the disease,” says Souza, who traveled to Bari to present her work on NAC in October 2014. According to the researcher, the European group has already started investigating the effect of the substance, with promising results. “NAC is absorbed by plants, but it remains to be seen whether it weakens the infection.”
In Apulia, Souza and Coletta-Filho looked at dry olive groves and talked to local residents, distressed by the loss of their trees. As they wait for an effective compound against the disease, they have resorted to spraying pesticides intensively in a protective belt around the afflicted area, in an effort to prevent infected leafhoppers from advancing towards the north, Italy’s most important olive oil producing region.
Project
Chemical and structural analysis of Xylella fastidiosa biofilms (nº 2010/51748-7); Grant Mechanism Regular Research Grant; Principal Investigator Mônica Alonso Cotta (Unicamp); Investment R$187,406.00 (FAPESP).
Scientific article
JANISSEN, R. et al. Spatiotemporal distribution of different extracellular polymeric substances and filamentation mediate Xylella fastidiosa adhesion and biofilm formation. Scientific Reports. V. 5, No. 9856. April 20, 2015.