It is well known that vaccines prevent healthy individuals from becoming severely ill when infected with the SARS-CoV-2 coronavirus, as was proven during the COVID-19 pandemic. But that is not all. Recent evidence indicates that the immunizations also protect against the severe form of the disease—reducing the risk of hospitalization and death—in individuals with inborn errors of immunity, genetic alterations that impair proper functioning of the immune system, previously known as primary immunodeficiencies. A new indication that vaccines actually benefit individuals with inborn errors of immunity was presented in March this year by researchers from the Federal University of São Paulo (UNIFESP) in an article published in the journal Frontiers in Immunology.
In the study led by pediatric infectious disease specialist Maria Isabel de Moraes-Pinto and immunologist Carolina Sanchez Aranda, medical student and PhD student Vitor Gabriel Lopes da Silva followed how 55 individuals with different types of inborn errors of immunity responded to the COVID-19 vaccine over a two-year period. They were between 13 and 61 years of age and received a primary vaccination series of three doses of the vaccine, followed by two booster shots. The performance of these individuals’ immune systems was compared to the immune response of 60 healthy participants from a similar age range, each of whom received four doses of the SARS-CoV-2 vaccine (two initial doses and two booster shots). Previous studies, with vaccines against other diseases, suggest that people with compromised immune systems should receive an additional dose to ensure adequate protection.
Lopes da Silva took part in a special research program at UNIFESP—the MD-PhD, which allows medical students to begin a PhD before graduating—and used two strategies to assess the performance of the immune system in the vaccinated participants. After each dose of the vaccine, he used a technique called microarray multiplex to measure the quantity of antibodies produced against specific segments of the virus—known as the humoral response. Using another test, the young researcher assessed the cellular response: he estimated the production of immune cells (especially T lymphocytes) and their ability to recognize the virus and act on the cells it had infected. According to the authors, this is the most comprehensive study to date to evaluate both the humoral and cellular responses in immunodeficient individuals to the largest number of SARS-CoV-2 variants (five), up to one month after completing a five-dose vaccination series.

Léo Ramos Chaves / Pesquisa FAPESP Healthcare team prepares vaccine against SARS-CoV-2, administered since May 2021 to people with inborn errors of immunityLéo Ramos Chaves / Pesquisa FAPESP
In general, vaccinated participants with inborn errors of immunity produced fewer antibodies than their healthy vaccinated counterparts. In particular, their bodies synthesized fewer neutralizing antibodies, those that prevent the virus from invading cells and causing infection in the body. This result was already somewhat expected, since seven of the eight immunodeficiencies assessed in the study were linked to failures in antibody production or in the functioning of the cells that synthesize them, the B lymphocytes. For this reason, 48 of the 55 participants with inborn errors of immunity received antibody replacement therapy. The immune response measured by T lymphocytes was similar in both groups. T lymphocytes are an extremely diverse set of immune cells: some varieties directly destroy infected cells; others signal other immune cells to carry out the destruction; and some stimulate B lymphocytes to produce antibodies.
“It is often presumed that immunodeficient patients will not respond to vaccines,” says Lopes da Silva. “Our study shows that even when producing few antibodies, these individuals can develop a good cellular response, which will probably help avoid severe forms of the disease,” explains the researcher, lead author of the article published in Frontiers in Immunology.
“Upon reviewing the study data, it becomes clear that the cellular response in these patients can, to some extent, compensate for the deficiency in antibody production,” says Moraes-Pinto, coordinator of the research and advisor to Lopes da Silva. “This is important because the role of the cellular response is often underestimated, even by healthcare professionals,” she adds.
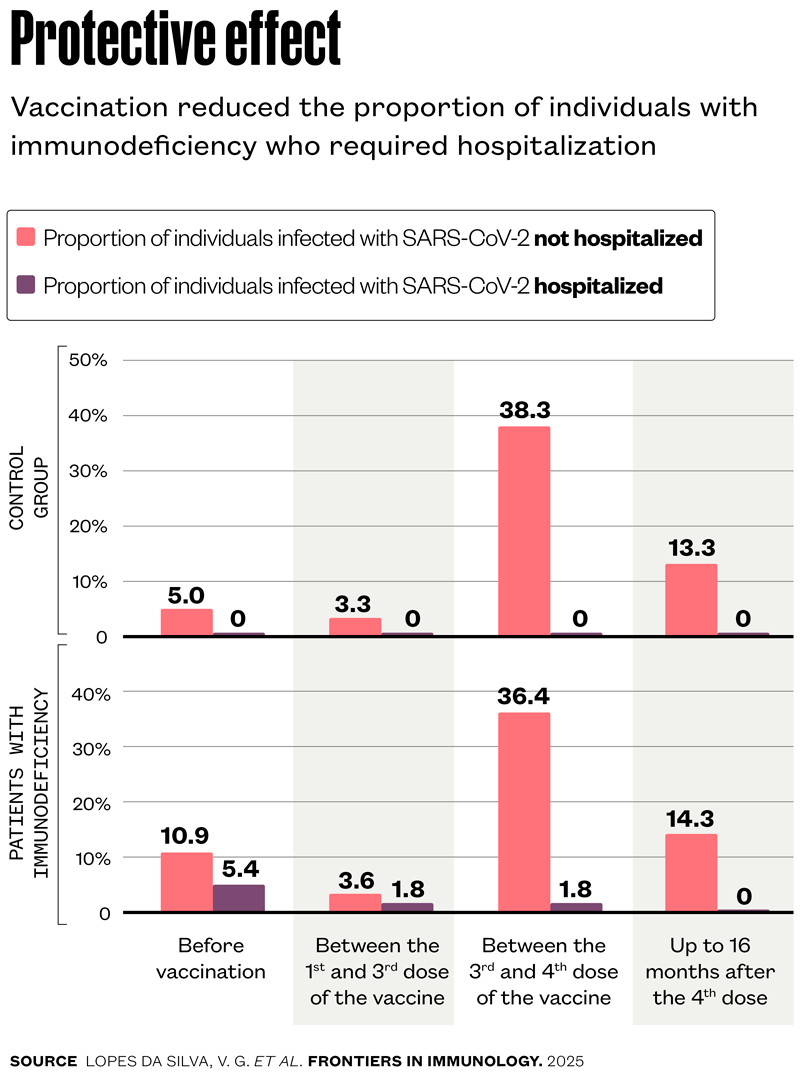
The most important effect observed by the UNIFESP group was the reduction in hospitalization rates as the vaccination series was completed. Before the start of immunization, which began in Brazil in May 2021 for people with inborn errors of immunity, nine of the 55 participants (16.3%) had already contracted COVID-19. Of these, three (5.4%) required hospitalization and six (10.9%) did not. The proportion of individuals requiring hospitalization fell to 1.8% after the first dose, even as the Omicron variant circulated throughout Brazil and infected nearly 40% of the participants. The hospitalization rate dropped to zero after the fourth dose. In the group of healthy participants, no one required hospitalization throughout the entire monitoring period (see graph below). “These data highlight the importance of the primary vaccination series with three doses for patients with inborn errors of immunity, as well as the need for booster shots every six months,” affirms Lopes da Silva.
“Studying patients with inborn errors of immunity allows us to better understand the mechanisms that lead to the development of the disease and identify which paths of the immune system can be activated to combat the virus,” says Aranda, PhD co-advisor to Lopes da Silva.
“The fact that patients with antibody production deficiencies develop a strong protective cellular response emphasizes the crucial role of T lymphocytes in protection against COVID-19,” affirms biologist and immunologist Cristina Bonorino, of the Federal University of Health Sciences of Porto Alegre (UFCSPA) and a member of the scientific and clinical committees of the Brazilian Society of Immunology (SBI), who did not participate in the current study.
Previous studies had already suggested that the cellular response could protect against severe illness. In an article published in 2022 in the journal Cell, the group of Italian immunologist Alessandro Sette, from the La Jolla Institute for Immunology, in the USA, had shown that T lymphocytes were capable of recognizing new coronavirus variants that escaped detection by antibodies induced by vaccination. At Massachusetts General Hospital, also in the USA, the team of physicians John Niles and Gaurav Gaiha found that vaccination reduced the risk of serious illness nearly fivefold in a group of patients with a specific form of immunodeficiency—common variable immunodeficiency—even in the absence of antibody production against SARS-CoV-2, according to an article published in 2023 in the journal Science Translational Medicine. “The results provide support for vaccinating this vulnerable population,” wrote the authors of the article.
“It’s important to recognize the merit of the team from UNIFESP for bringing together this diverse cohort of patients with inborn errors of immunity, which is quite challenging,” says immunologist Heldar Nakaya, of the Hospital Israelita Albert Einstein Teaching and Research Institute and the School of Pharmaceutical Sciences at the University of São Paulo (FCF-USP), who did not participate in the work published in Frontiers in Immunology. He stresses, however, that future studies should work with a more homogeneous group of patients in terms of diagnosis, which would allow more accurate comparisons with the control group. In his opinion, an interesting extension would be to apply single-cell transcriptomic techniques to the material collected from these patients to identify which cells are being activated and which genes are expressed in response to vaccination.
The UNIFESP group now plans to monitor the long-term evolution of the immune response in patients with inborn errors of immunity and investigate the role of immunoglobulin (antibodies) replacement therapy in the performance of vaccines.
The story above was published with the title “Beyond antibodies” in issue 353 of July/2025.
Project
Prospective study of humoral and cellular immune response after immunization against COVID-19 in patients with inborn errors of immunity (IEI) (n° 21/13419-6); Grant Mechanism Regular Research Grant; Principal Investigator Carolina Sanchez Aranda Lago (UNIFESP); Investment R$275,930.85.
Scientific articles
LOPES DA SILVA, V. G. et al. Enhanced T-cell immunity and lower humoral responses following 5-dose Sars-CoV-2 vaccination in patients with inborn errors of immunity compared with healthy controls. Frontiers in Immunology. Mar. 6, 2025.
TARKE, A. et al. Sars-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. Jan. 24, 2022.
ZONOZI, R. et al. T cell responses to Sars-CoV-2 infection and vaccination are elevated in B cell deficiency and reduce risk of severe Covid-19. Science Translational Medicine. Nov. 29, 2023.
