
James Gathany / CDC A double risk: the mosquito Aedes aegypti…James Gathany / CDC
Dressed like a surgeon, the researcher Stella Melo worked in total silence in a biosafety laboratory at the University of São Paulo (USP) on the afternoon of Friday, December 11, 2015. Inside a booth in which only filtered air circulates, she seeded monkey kidney cells into plastic bottles containing a pinkish nutritional liquid. Although she wore a mask, she avoided talking to avoid the risk of contaminating the material. Days later those cells would serve to reproduce the Zika virus, an infectious agent that for decades was considered harmless and is now alarming Brazil and the rest of the world. It is suspected that the virus is associated with babies born with smaller heads than normal, a problem without a cure known as congenital microcephaly.
The following Thursday, December 17, the virologist Danielle Leal de Oliveira used some of the cells prepared by Melo to start a Zika culture and announced in an e-mail: “I inoculated the viruses today. We have our fingers crossed to see if they grow.” Oliveira and Melo are part of the Edison Durigon team of virologists at the Institute of Biomedical Sciences at USP (ICB/USP). They worked hard to replicate the Zika samples received from the Evandro Chagas Institute, located in Pará State. The goal was to multiply the virus and share it with groups in Brazil and abroad that planned to study it. There was no lack of interest.
Since Zika gained worldwide importance in November 2015 with reported cases of microcephaly, the virologist Paolo Zanotto, a Durigon colleague and their next-door neighbor at USP, thinks of nothing but containing the virus. Zanotto, who is a specialist in the evolution of flaviviruses, the group to which Zika belongs, knows the enormous risk of its spreading across Brazil—particularly in the state of São Paulo, where Zika’s transmitter, the mosquito Aedes aegypti, is found throughout the urban population. He also knows that the only way to contain the Zika virus is with a coordinated effort by researchers, the government and the population.
For this reason, as early as November 2015, Zanotto began to mobilize the virologists, epidemiologists, doctors and entomologists of São Paulo and abroad to study everything possible about Zika. In late December, 32 groups from São Paulo (nearly 300 researchers) agreed to form a network to investigate the virus—it was informally dubbed the Zika Network—and several groups awaited the virus samples from the Durigon laboratory to start the research.

Fred Murphy and Sylvia Whitfield / CDC… and copies of the virus (dark spots) of the Flaviviridae family, the same as ZikaFred Murphy and Sylvia Whitfield / CDC
This prompt reaction was possible because, in the past, FAPESP has supported the creation of virology laboratories across the state of São Paulo that are characterized by strong interaction among them. Many of them have thematic projects or regular research grants from the Foundation, and to reactivate the collective work of the group, FAPESP provided additional funding to existing projects. This additional funding will total approximately R$550,000 and will complement the work already being done.
Jean Pierre Peron is a neuroimmunologist working at USP’s laboratory. Among other areas of interest, he studies inflammation in the brain caused by the body’s own immune system. Peron is part of the Zika Network, and he and his team are ready to start at least two experiments. In one of the experiments, he plans to inject the virus directly into the brains of mice, having two goals in mind. The first is to let it multiply and generate more samples for his research and that of the other groups. The second, and more important goal, is to see if the virus itself harms the brain or if the damage arises from an exacerbated attack against Zika by the immune system.
Brain scans of babies born with microcephaly whose mothers were possibly infected with Zika during pregnancy, in general, show small white circles very close to each other, like the beads of a necklace. According to neurologists, they are signs of calcification, a type of scar that forms in damaged areas of the brain, and also occurs in infants whose mothers had cytomegalovirus infection or toxoplasmosis during pregnancy. In the case of Zika, it is unknown whether these calcifications are caused by the virus or are a secondary injury, the result of a super-attack by immune cells against the invader.
It is also not yet known how the virus reaches the brain, as noted in a baby born in Ceará State with microcephaly and who died within minutes after birth. It was from various tissue samples of this baby that the virologist Pedro Vasconcelos and his team were able to isolate the Zika samples sent to São Paulo. They work at the Evandro Chagas Institute, which is Brazil’s national virology reference center. The leading suspicion is that the virus develops better in nervous system cells, which is also true of almost 60 others of the Flaviviridae family, the same family as the dengue and yellow fever virus.
 A second experiment planned by Peron may help to confirm Zika’s preference for brain tissue cells and to trace the path taken by the virus to reach the central nervous system. He and his team are ready to inoculate the virus into pregnant female mice and monitor what happens to the fetuses. “This will let us see if the virus reaches the brain of the fetus and causes injury, death or microcephaly,” Peron said on a visit to Durigon’s laboratory on the afternoon when Melo was preparing the cells for Zika to multiply.
A second experiment planned by Peron may help to confirm Zika’s preference for brain tissue cells and to trace the path taken by the virus to reach the central nervous system. He and his team are ready to inoculate the virus into pregnant female mice and monitor what happens to the fetuses. “This will let us see if the virus reaches the brain of the fetus and causes injury, death or microcephaly,” Peron said on a visit to Durigon’s laboratory on the afternoon when Melo was preparing the cells for Zika to multiply.
Peron’s work with mice will be complemented by the experiments of biologist Patrícia Beltrão Braga using human cells. “The first thing we need to know is if, in fact, the virus infects the human cells of the nervous system and what type of cell death it causes,” says Braga. Based on information circulating among researchers and by extrapolating what is known about other flaviviruses, Zika is likely to be invading brain tissue, but it is not known which cells or how. This information may in the future guide physicians as to which therapy to prescribe to contain the virus and the damage it causes, but for now, however, there is no safe drug to combat Zika.
Braga will analyze the effects of the virus on human cells using an innovative technology. She will use adult stem cells taken from children’s baby teeth and chemically reprogram them to transform them into more versatile cells, capable of generating different types of tissue. Cultured in a three-dimensional matrix, these cells, upon receiving certain chemical stimuli, produce different types of central nervous system cells and are organized into layers, as if they were microscopic brains—some only the size of a pinhead.
Braga plans to infect the mini-brains with Zika and track their changes. “My idea is to assess whether the virus impairs cell growth, protein production and the formation of synapses, which are the connections between neurons,” she says. “I think the mini-brains should give us a quick answer to some questions,” says Braga, who attended the first meeting of the Zika Network in early December 2015. At that point, the Ministry of Health had recorded the presence of the virus in 18 states, mainly in the Northeast, where the first cases were identified. And the virus could spread even further.
 One of the difficulties of planning effective actions to contain the virus is that its circulation pattern among the Brazilian population—or in other populations for that matter—is still unknown. No one knows how many people have been infected in Brazil or how many new cases arise each month. There is also no data on the infection rate of mosquitoes and their efficiency in transmitting the virus through bites. “If we had this information, we could calculate the infection’s capacity to spread,” says epidemiologist Eduardo Massad, of USP’s School of Medicine, who has also joined the network.
One of the difficulties of planning effective actions to contain the virus is that its circulation pattern among the Brazilian population—or in other populations for that matter—is still unknown. No one knows how many people have been infected in Brazil or how many new cases arise each month. There is also no data on the infection rate of mosquitoes and their efficiency in transmitting the virus through bites. “If we had this information, we could calculate the infection’s capacity to spread,” says epidemiologist Eduardo Massad, of USP’s School of Medicine, who has also joined the network.
One way of getting to know these variables is to record cases of infection in real time, to see how they evolve in time and space. One tool to do this would be a reliable laboratory test to identify old Zika infections and learn where the virus has already been and when. The current way of doing this type of tracking is through serological tests, which detect antibodies against the virus in the blood. This type of test reveals whether or not an infection is old or new, but it does not work well in the case of Zika. The antibodies against it are actually similar to those generated against the dengue virus, which occurs throughout Brazil.
An alternative way to determine the infection, now available in nearly 20 laboratories of Brazil’s public health system, is a test that relies on the polymerase chain reaction (PCR). It amplifies a region of genetic material of the virus, but the test is more complex and requires expensive equipment and trained personnel. Also, it is only able to detect the Zika virus once the infection is active and the person has symptoms.
Since most of the Zika Network laboratories already have equipment to perform PCR—many are former members of the Virus Genetic Diversity Network (VGDN), a program funded by FAPESP from 2000-2005—Zanotto plans to leverage this capacity to assist in monitoring Zika in the state of São Paulo. The idea is for these laboratories to perform a molecular diagnosis of people suspected of being infected. This would enable monitoring of the infection’s progress, almost in real time, and assist the epidemiological surveillance services in combating outbreaks of active infection.

LÉO RAMOSStella Melo microscopically analyzes cell cultures at USPLÉO RAMOS
There are plenty of reasons for the urgency. Summer has begun and with it the rainy season in the Southeast, home to 82 million people, or four out of every 10 Brazilians. Virologists, epidemiologists and public health experts fear that Zika will find fertile ground in which to thrive. The virus is injected into humans by the bite of female Aedes aegypti, a dark mosquito with white striped legs that usually feeds on blood during the day. In addition to feeding on blood, the mosquito needs only a small amount of standing water to spawn its offspring. And over a period of a few years, it has become resistant to insecticides (see Pesquisa FAPESP Issue nº 147).
Another reason for concern is that Aedes aegypti, which also transmits the virus for dengue, yellow fever and chikungunya fever, has already spread to the Southeast. The most striking evidence of the mosquito’s presence is the number of dengue cases in 2015. Last year the Ministry of Health identified 1.6 million suspected cases of infection in Brazil, of which 990,000 or 61% occurred in the Southeast (718,000 in the state of São Paulo). Some researchers are suggesting that many of these mosquitoes are already carriers of Zika.
It has been known for some time that Zika is circulating, even though tentatively, in Brazil’s Southeast. The states of São Paulo, Rio de Janeiro and Espírito Santo by late November 2015 recorded a few localized cases that were confirmed by molecular tests. But there was no official and accurate count.
The first case in São Paulo was detected on May 19, 2015 when the Adolfo Lutz Institute, one of Brazil’s reference laboratories for viral detection, confirmed the presence of Zika in the blood of a 52-year-old male resident of Sumaré, in the Campinas region. Another case was recorded in São José do Rio Preto, in the state’s northwest, and two more in Ribeirão Preto, in the north. “It is possible that Zika has been circulating for a few months in the state, but is not widespread,” says virologist Marcos Boulos, head of the Coordinating Bureau for Disease Control of the São Paulo State Department of Health. “Otherwise, we would have confirmation of neurological problems,” he said on December 14, before the Departmental Secretary, David Uip, announced that six babies with microcephaly were being examined for Zika infection.

LÉO RAMOSMonkey kidney cells infected with the Zika virusLÉO RAMOS
The size of the problem is still unknown. In mid-December the Ministry of Health published a document in which it projected, but with high degree of uncertainty, the number of infected people in Brazil. Between 443,000 and 1.3 million Brazilians may have had Zika, a disease that is confused with dengue, but in 80% of the cases there was no apparent sign or cause, just a passing malaise at most (see table). The authors of the document arrived at these figures by using estimates from the international medical literature and numbers of suspected dengue cases unconfirmed by laboratory tests.
The virologist Maurício Nogueira Lacerda, a physician and professor at Rio Preto Medical School, is one of those who suspects that some cases identified as dengue were, in fact, Zika. For almost a decade he has been following dengue outbreaks in São José do Rio Preto and in April and May of 2015 he found something unusual: cases of Guillain-Barré syndrome, an inflammatory nerve-degenerating disease, in people with symptoms of dengue. “In retrospect, they may have been Zika,” he says. He will soon begin testing for the virus on about 300 blood samples from early 2015 classified as dengue—Zanotto plans to do the same with another 1.2 billion from São Paulo.
If the virus has been in São Paulo for longer and is, in fact, the cause of microcephaly, new cases may appear soon. “The dengue peak in São Paulo and therefore the peak of Aedes aegypti circulation occurred between April and May 2015, and women who were pregnant at the time are about to give birth,” says Lacerda. He and his group must monitor 2,200 people for five years to find the percentage of asymptomatic cases of Zika, and the risk of microcephaly in babies of pregnant women infected with the virus.
The suspicion of Zika’s connection to microcephaly, something unheard of in the world, appeared in October 2015. One month before that, the pediatric neurologist Vanessa Van Der Linden had begun noticing an unusual increase in the number of cases of microcephaly at the Hospital Barão de Lucena, where she works in Recife (capital of Pernambuco State), and she notified the Pernambuco State Department of Health. Then, researcher Carlos Brito, of the Federal University of Pernambuco, suggested that Zika could be behind the cases, and the problem was reported to Brazil’s Ministry of Health, which notified the World Health Organization.
The strongest evidence came only in late November 2015, when Vasconcelos was able to isolate the virus of the Ceará baby and the Oswaldo Cruz Foundation confirmed the presence of Zika in the amniotic fluid of two pregnant women from Paraíba whose fetuses had microcephaly. By December 15, the Ministry had confirmed 134 cases associated with the Zika infection—in Pernambuco, Paraíba, Rio Grande do Norte and Sergipe—and discarded 102. Another 2,165 remained under investigation.
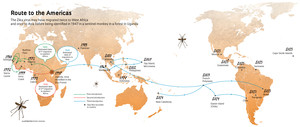
Several experts consulted by the report agreed that Zika was the prime suspect as the cause of microcephaly. In addition to the temporal connection between the two problems, the virus appears to be adapting to infect humans. In a study done by researchers at the Pasteur Institute in Senegal, the biomedical scientist Caio Melo Freire, of the Federal University of São Carlos, showed that the strain in circulation in Brazil came from Africa via Asia (see map). Along the way, the virus humanized: some of its genes have the recipe to make proteins more similar to those of human genes.
Still, some researchers say that more data are needed to answer the question. “We do not know, for example, if the vulnerability of the fetus is restricted to the first trimester or if it also comes later and leads to other problems,” says Fernando Kok, a USP neurologist. “The causal relationship is plausible and the signs are strong,” says Celso Granato, an infectious disease specialist at the Federal University of São Paulo (Unifesp). “But we need to have more thoroughly studied cases, because there may be other co-factors that we have yet to discover.”
“If you asked me if Zika causes microcephaly, I would say I don’t know,” said epidemiologist Eduardo Massad in early December. He has many questions as yet without answers. “Now if causation is proven,” he added, “Zika could become the Godzilla of infections.”
Scientific articles and other documents
FAYE, O. et al. Molecular evolution of Zika virus during its emergence in the 20th century. PLOS Neglected Diseases. January 9, 2014.
FREIRE, C.C.M. et al. Spread of the pandemic Zika virus lineage is associated with NS1 codon usage adaptation in humans. Biorxiv.org.
ZANLUCA, C. et al. First report of autochthonous transmission of Zika virus in Brazil. Memórias do Instituto Oswaldo Cruz. June 11, 2015.
CAMPOS, G.S.; BANDEIRA, A.C.; SARDI, S.I. Zika virus outbreak, Bahia, Brazil. Emerging Infectious Diseases. October 2015.
Surveillance and response protocol to the occurrence of microcephaly related to infection by the Zika virus – http://bit.ly/1REOZ2w.