Some experts believe that hunger and satiety are two extremes of a physiological state that controls the pursuit of essential energy to keep the body alive and functioning in balance. Like a scale, the desire to eat and the feeling of satiety fluctuate throughout the day, regulated by a series of substances produced by the digestive system and adipose tissue, which inform the central nervous system of the availability of energy or the need to look for food. In the brain, hunger and satiety are governed by a deep and multipurpose structure: the hypothalamus. Smaller than an almond and roughly cone-shaped, the hypothalamus serves as a sort of central caretaker for the body. In addition to controlling the desire to eat, it directly or indirectly regulates temperature, thirst, fatigue, sleep, social bonding, and libido.
Papers published this year by two Brazilian groups are shedding light on some of the pieces of the biochemical puzzle that influence feelings of hunger and satiety in the hypothalamus, whose functioning had hitherto gone unnoticed by science. One of them, in fact, seems to be a potential target for drugs designed to control exaggerated weight gain, an issue that has plagued the entire planet in recent decades. Today, being overweight and obesity affect just over half of the world’s population—which is why this epidemic was named globesity.
In experiments with mice conducted at the University of Campinas (UNICAMP), the team led by immunologist Lício Velloso found that, by increasing the expression of a single gene, active only in a small number of cells in the hypothalamus, it may be possible to reduce weight gain, since it stimulates satiety and energy expenditure, in addition to alleviating signs of anxiety and depression, frequently experienced by a significant portion of overweight and obese individuals. At the University of São Paulo (USP), physiologist José Donato Junior and his collaborators found, also while experimenting with rodents, that a hormone called ghrelin, produced in the digestive system, and traditionally associated with hunger, does not act alone. It needs the simultaneous action of another compound—growth hormone, which has hitherto been exclusively associated with the increase of energy consumption—to trigger the food-seeking impulse in the hypothalamus.
Rodrigo Carraro / Unicamp
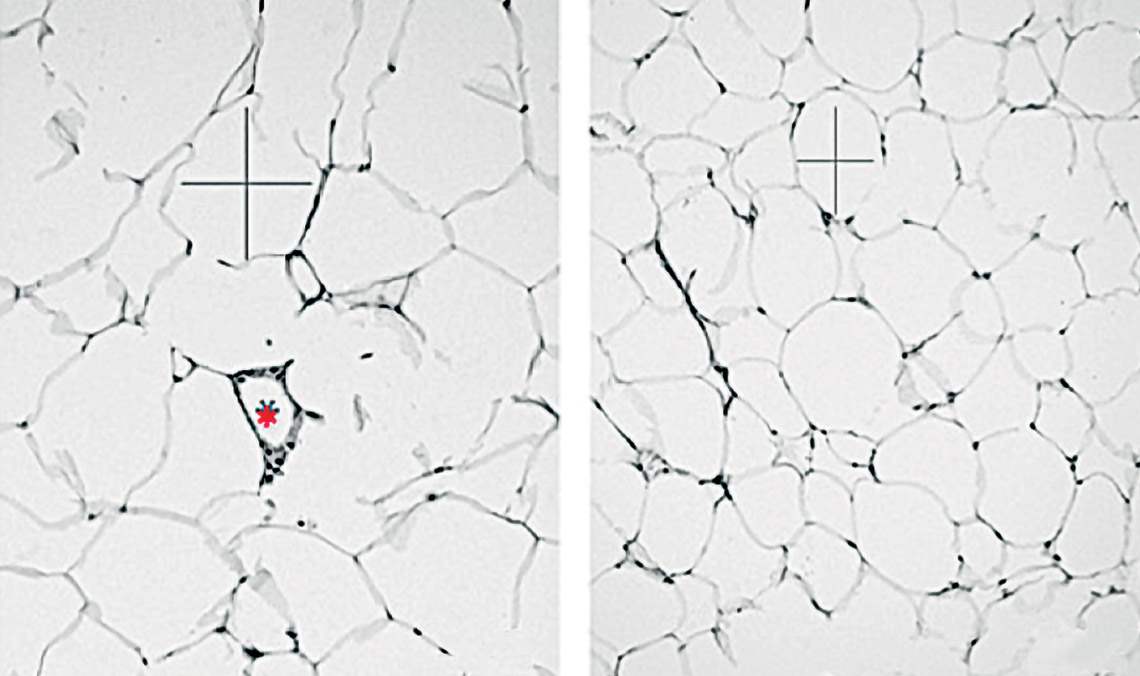
Microscopic image of adipose tissue cells, which are smaller and fewer in animals whose NHLH2 gene is more active (right) than in the control groupRodrigo Carraro / UnicampThese papers, alongside others conducted in Brazil and abroad, help us figure out the intricacies of the mechanism that controls satiety and hunger and the challenges in changing its functioning without causing significant damage. “The mechanisms that control energy balance are redundant and complex,” says biochemist Marcio Torsoni, from UNICAMP, who was not a part of either study. “For this reason, any hormonal interference for the treatment of obesity can only be performed after a perfect understanding of how each hormone affects cell signaling and its repercussions on other metabolic and behavioral events,” he adds.
A peculiar characteristic makes the gene studied by Velloso’s group a good target for the action of drugs that fight weight gain. The nescient helix-loop-helix 2 (NHLH2) gene—given such a complicated name due to the structural characteristics of the protein it encodes and its function—is normally expressed in a very restricted group of cells in the hypothalamus: the neurons that produce pro-opiomelanocortin, known by the acronym POMC. When they are full of fat, the adipose tissue cells distributed throughout the body release the hormone leptin into the bloodstream, which tells the brain it is time to stop eating. In the hypothalamus, leptin activates POMC neurons and these, in turn, release a neurotransmitter that activates other neurons and induces satiety.
An increase in the expression of the NHLH2 gene reduced weight gain and increased energy expenditure
It has been known for over two decades that NHLH2 is somehow associated with weight gain in animals and humans. In 1997, in a study to investigate the function of this gene during a postdoctoral internship at the National Cancer Institute in the United States, biomedical scientist Deborah Good disabled the two copies of the gene in mouse embryos and observed what happened. Without NHLH2, rodents did not adequately develop sexual organs, lost their libido, and became obese as adults. Years later, she also found that these animals were less active than usual.
“These and other studies that disabled or reduced the expression of the gene pointed to a decline in the obesity issue,” says Velloso, coordinator of the Obesity and Comorbidities Research Center (OCRC), one of the Research, Innovation, and Dissemination Centers (RIDC) funded by FAPESP. “It was clear that it was important for weight regulation, but we had not yet tried to verify what happens when its activity is increased,” explains the immunologist.
Rodrigo Carraro / Unicamp
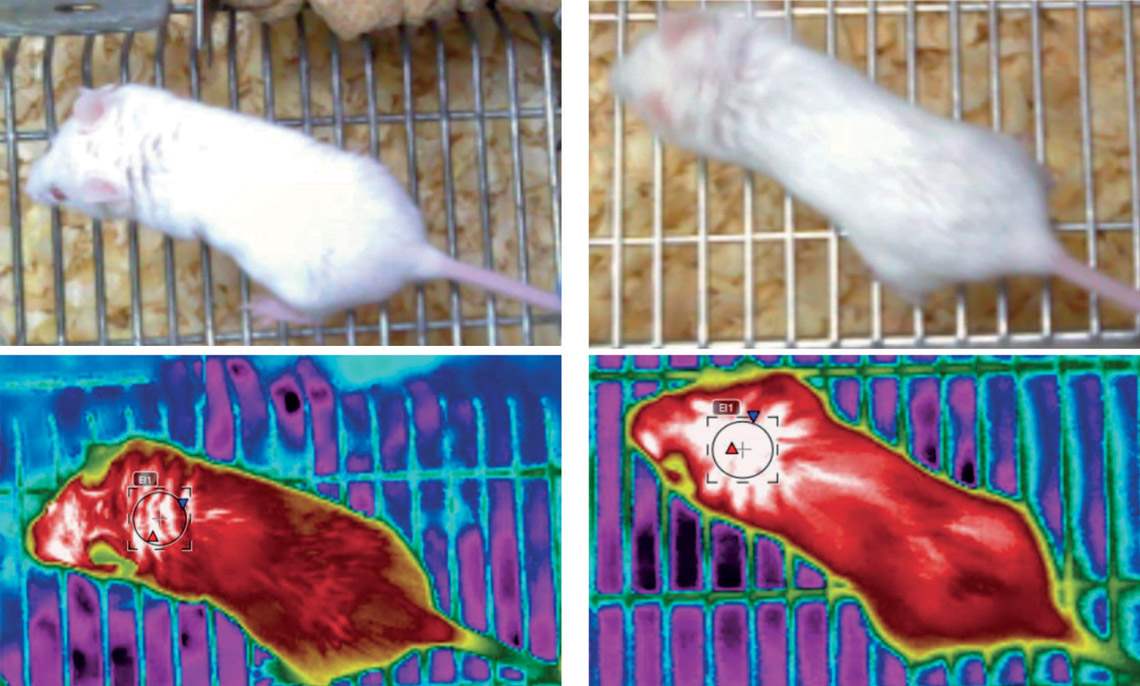
Mice whose NHLH2 gene had a more significant expression (right) had a body temperature (measured at the nape of the neck) around 1.3 oC higher than animals in the control groupRodrigo Carraro / UnicampWhen obtaining his PhD under the supervision of Velloso at the UNICAMP Laboratory of Cell Signaling, biologist Rodrigo Carraro used a genetically modified virus to increase the expression of the gene in POMC neurons by 40%. In simple terms, the increased expression of this gene caused a preventive and a curative effect. In animals that were at a healthy weight at the beginning of the tests and were fed a high-fat (hyper-lipid) diet, which causes obesity, the increased activity of NHLH2 prevented them from becoming obese by increasing satiety. The rodents that produced more of the protein encoded by the gene ate less than those with normal NHLH2 activity and gained about 40% less weight, according to results published in late October in the Journal of Neuroscience.
In mice that were already obese at the beginning of the experiment, the effect was even greater. Also fed a high-fat diet, they gained 80% less weight than the control group. They also burned 15% more energy by being physically more active and producing more heat in brown adipose tissue. They walked more in their cages and exercised longer on the treadmill or wheel. Their body temperature was also on average 1.3 degrees Celsius (°C) higher than that of animals with normal gene activity. The average temperature in the first group was 34.3 °C, compared to 33 °C in the second group. In the behavioral experiments, mice that synthesized more of the protein encoded by NHLH2 showed fewer signs of depression and anxiety.
“We had not yet associated such crucial functions as controlling hunger and reducing depression and anxiety to this gene,” says Velloso. If compounds capable of increasing the expression of NHLH2 in the hypothalamus can be produced in the future, individuals who are anxious and obese would benefit most from it, researchers presume. They tend to be more sensitive to minor changes in their environment or routine, which leads them to look for food even when not hungry.
The action of ghrelin—the so-called hunger hormone—is not enough to arouse appetite
“The hypothalamus controls a multitude of physiological processes at the peripheral and central levels and is connected with areas of the brain related to behavior, among other things. The link between obesity and anxiety or depression is well known in humans and is associated with overeating. This paper provides a new molecular basis, hitherto unknown, for this association,” states physiologist Miguel Antonio López, from the University of Santiago de Compostela in Spain, who studies the role of hypothalamic neurons in the perception of energy levels in the body.
The UNICAMP team now plans to start a trial of compounds that have already been synthesized in order to find some that might have an affinity for the gene and increase its expression, thus increasing satiety. If one of these molecules works in humans, it can result in a drug treatment with limited adverse effects, as NHLH2 is expressed in a very restricted number of neurons. “This gene is a good target, because it’s in the right neuron,” says Velloso.
The USP team, on the other hand, has not yet had the same luck in finding such a specific target, although their work is shedding light on how hunger works. Since its discovery in 1999, ghrelin has been considered the main factor in provoking the urge to eat, which is why it has been called the “hunger hormone.” Produced in the stomach and intestines, it acts on another small group of cells in the hypothalamus: the AgRP/NPY neurons, so called because of the two neuropeptides they produce. Its effect is so powerful that, when administered in the bloodstream, it makes people or animals look for food even after a large meal.
Frederick Wasinski / USP
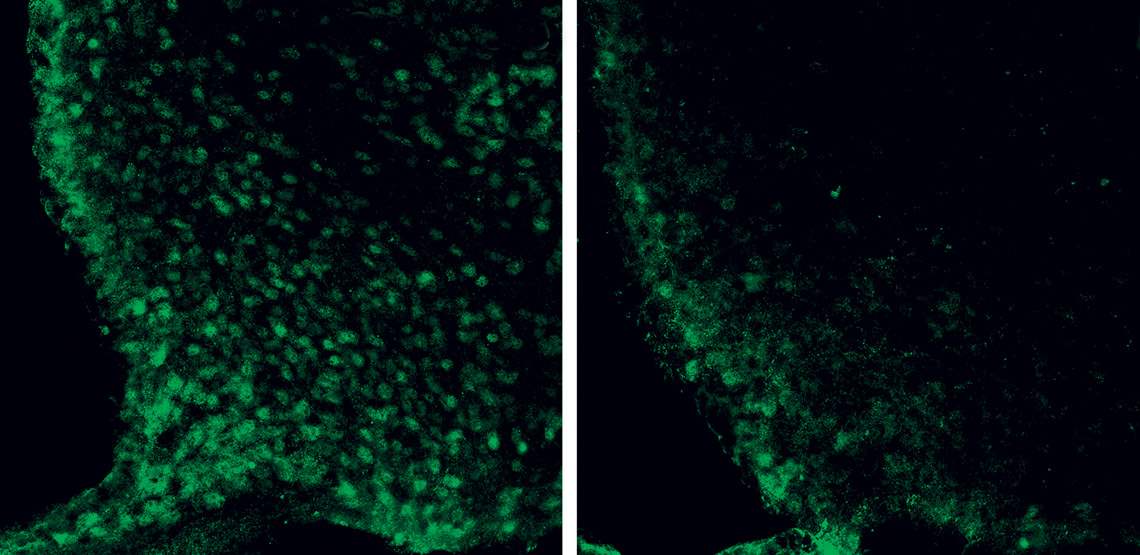
The microscopic image shows neurons in the hypothalamus (green circles) of mice whose growth hormone (GH) receptor was intact (left) and disabled (right)Frederick Wasinski / USPGhrelin began to prove more complicated after it was discovered, a few years ago, that it also acts in the pituitary, a gland adjacent to the hypothalamus, stimulating the production of growth hormone (GH). In peripheral organs and tissues, this compound stimulates cell proliferation and energy expenditure, leading to growth in the initial stages of life and tissue repair in adults. In 2019, however, the USP group found that, during fasting, this hormone also awakens AgRP/NPY neurons and stimulates hunger, while signaling to the body that it needs to save energy (see Pesquisa FAPESP issue nº 277).
“For some time, it was speculated that the effect of ghrelin and that of GH occurred in parallel and independently,” explains Donato. His team’s work now indicates that the isolated action of ghrelin on AgRP/NPY neurons is not enough to cause hunger. It depends on the simultaneous action of GH in the hypothalamus, possibly affecting another group of neurons.
This relationship became evident in the experiments carried out by physiologist Frederick Wasinski from 2016 to 2020, during a postdoctoral internship supervised by Donato. At the USP Functional Neuroanatomy Laboratory, Wasinski produced two types of genetically altered mice, each with a different degree of insensitivity to the action of GH. The animals in the first group lacked the molecule (receptor) to which this hormone binds on the surface of the neurons. In the second group, these receptors were absent only on AgRP/NPY neurons. In both cases, the rodents were still able to produce growth hormone normally and ate, proportionally, the same amount of feed as the animals in the control group, whose receptors were intact and active.
Differences began to appear in two tests involving food restriction, when there is a natural increase in the production of ghrelin and GH. In the first experiment, the animals spent 24 hours without access to food, before being allowed to eat freely. In the second experiment, they were allowed to eat freely after five days of food restriction (in which they got 60% of the calories they needed). In both conditions, the animals lacking the GH receptor in the brain ate more after the period of food deprivation, but still at a much lower level than the control group. This indicated that, without the action of growth hormone, ghrelin did not arouse hunger as before.

In another set of tests, Wasinski artificially stimulated hunger (by injecting a dose of ghrelin into the bloodstream) and found that mice lacking the GH receptor only on AgRP neurons started to eat as much as those in the control group, in which this hormone was unchanged. The mice that lacked the receptor in the whole brain ate less food than the other two groups, according to data published in May in Endocrinology. Days later, the group led by endocrinologist Jeffrey Zigman, from the University of Texas Southwestern Medical Center, USA, presented a similar conclusion in a Molecular Metabolism article, in which he describes tests carried out with rodents that did not produce growth hormone, because they lacked the ghrelin receptor in the pituitary gland.
According to the USP researchers, this result suggests that appetite stimulation by ghrelin depends on the concomitant action of growth hormone in other groups of neurons in the hypothalamus, and not just on AgRP/NPY. Currently, Donato’s team is conducting other tests to identify what these neurons are.
“From a physiological standpoint, the synergistic action of the two hormones makes a lot of sense, since the effect of growth hormone depends on the supply of energetic nutrients, facilitated by the action of ghrelin to stimulate hunger,” states Torsoni, from UNICAMP. “Knowing how their interaction modulates the activity of different populations of neurons in the hypothalamus is essential to identify which of them could be manipulated by possible drugs to treat obesity,” he concludes.
Eduardo Ropelle, a physiologist at UNICAMP, who was not part of this research, believes the results obtained by the USP group may open new therapeutic possibilities for the treatment of obesity, since compounds that prevent the action of ghrelin are being extensively tested in experimental models, with modest and often inconclusive results. “The combination of compounds capable of selectively inhibiting both ghrelin and GH could present more promising results, although the risk of adverse effects cannot be disregarded,” says Ropelle.
Projects
1. Assessment of the expression and distribution of the NHLH2 protein in the hypothalamus of animals submitted to a high-fat diet (nº 16/00977-2); Grant Mechanism Doctoral (PhD) Fellowship; Supervisor Licio Augusto Velloso (UNICAMP); Grant Beneficiary Rodrigo Scarpari Carraro; Investment R$129,997.69.
2. OCRC – Obesity and Comorbidities Research Center (nº 13/07607-8); Grant Mechanism Research, Innovation and Dissemination Centers (RIDCs); Principal Investigator Licio Augusto Velloso (UNICAMP); Investment R$44,752,532.26.
3. The central nervous system as a growth hormone target for the regulation of multiple biological functions (nº 20/01318-8); Grant Mechanism Thematic Project; Principal Investigator José Donato Junior (USP); Investment R$1,839,220.10.
4. The role of orexin neurons as mediators of growth hormone-induced central effects (nº 16/20897-3); Grant Mechanism Postdoctoral Fellowship; Supervisor José Donato Junior (USP); Grant Beneficiary Frederick Wasinski; Investment R$297,822.48.
5. The effects of growth hormone on POMC, cholinergic, and paraventricular nucleus neurons of the hypothalamus: implications on the control of energy and glycemic metabolism (nº 17/25281-3); Grant Mechanism Postdoctoral Fellowship; Supervisor José Donato Junior (USP); Grant Beneficiary Paula Gabriele Fernandes Quaresma Bergonsi; Investment R$201,727.01.
Scientific articles
CARRARO, R. S. et al. Arcuate nucleus overexpression of NHLH2 reduces body mass and attenuates obesity-associated anxiety/depression-like behavior. Journal of Neuroscience. Oct. 21, 2021.
WASINSKI, F. et al. Ghrelin-induced food intake, but not GH secretion, requires the expression of the GH receptor in the brain of male mice. Endocrinology. May 10, 2021.
GUPTA, D. et al. Disrupting the ghrelin-growth hormone axis limits ghrelin’s orexigenic but not glucoregulatory actions. Molecular Metabolism. May 21, 2021.
