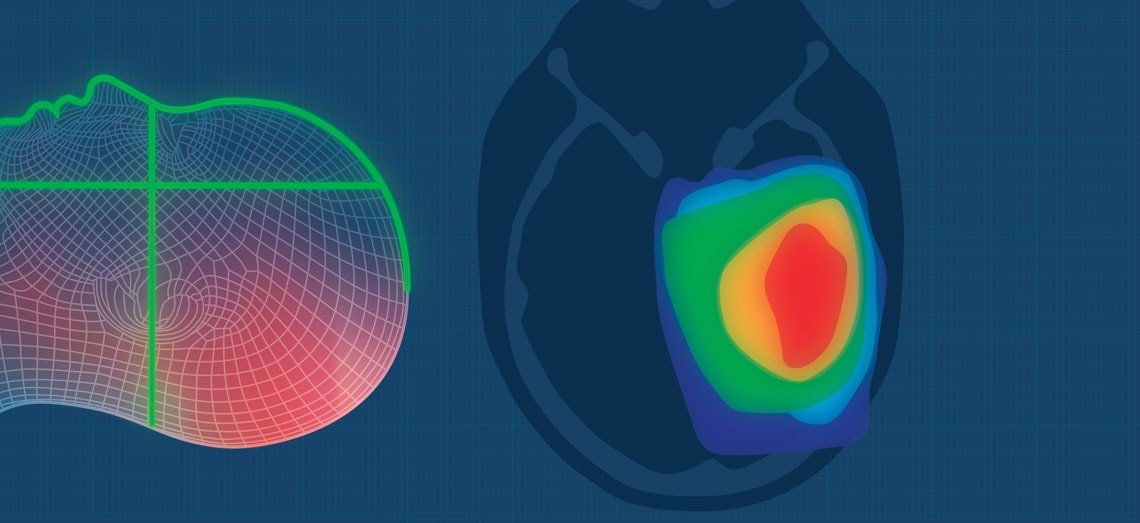
A new Radiation Treatment Planning System (TPS) developed by i-Medsys, a medical software development firm in Ribeirão Preto, São Paulo, in southeastern Brazil, promises to deliver more efficient and safer radiation therapy for cancer in developing countries. The software suite, dubbed Siprad, is currently under approval review by the Brazilian Health Regulatory Agency (ANVISA) and the US Food and Drug Administration (FDA).
Radiation treatment planning, a stage involving a multidisciplinary team of radiation oncologists, medical dosimetrists, and medical physicists, consists of determining the dose and the different angles of radiation delivered from a machine outside the body—known as a linear accelerator—and delineating the target volumes in the body that will receive treatment in order to enhance effectives and reduce side effects. “Planning is the most critical stage of radiotherapy treatment,” says radiation oncologist Harley Francisco de Oliveira, vice chairman of the Brazilian Society for Radiotherapy, noting that an improperly delivered dose can cause undesirable damage to healthy tissues.
Without radiation treatment planning systems like Siprad, physicians define the target region and radiation dose through visual observation of computerized tomography or magnetic resonance imaging scans, increasing the risk of error. This is the situation in most radiation therapy services within the Brazilian National Healthcare System (SUS). According to data from the Brazilian Ministry of Health, 10.3 million radiation therapy procedures were performed within the SUS in 2017. A shortage of radiotherapy equipment, such as linear accelerators, and the absence of TPS systems means that patients wait an average of 113 days from diagnosis before they start radiation therapy.
But implementing radiotherapy technology is expensive, and access to treatment is limited outside Brazil’s major hospitals. A linear accelerator costs approximately US$1.5 million (around R$5.5 million). A TPS software license from US-based Varian, the global market leader alongside Swedish Elekta, can cost from US$10,000 to upward of US$100,000, says the company’s country manager for Brazil, Humberto Izidoro. The total price depends on what features are configured and the number of registered users for each feature. A clinic or hospital also requires a dedicated workstation to operate the TPS.
Diego Fiori de Carvalho, a managing partner at i-Medsys, says the value proposition for Siprad is a lower total cost of ownership. A full-featured license for the system—which was developed with funding support from the FAPESP Technological Innovation in Small Businesses (PIPE) program—costs approximately US$10,000, the same price as for a Varian license for a single feature and one user. Another differentiator is that whereas imported TPS software typically requires a dedicated workstation, Siprad can run on a personal computer. The Brazilian-developed software, says Carvalho, offers the same features as competing systems and is compatible with linear accelerators produced by both Varian and Elekta.
Siprad was developed in response to demand for radiation therapy at a university hospital (HCRP) run by the Ribeirão Preto School of Medicine at the University of São Paulo (USP), then headed by Harley Oliveira. “The high cost of TPS licenses placed a constraint on expanding radiotherapy services, especially for patients relying on government funding,” says the physician. i-Medsys, a startup incubated at the SUPERA Innovation and Technology Park in Ribeirão Preto, had previously supplied HCRP with a picture-archiving and communication system, called LyriaPacs, which was also developed with funding from FAPESP.
The demand for treatment planning systems at HCRP led i-Medsys owners Diego Carvalho and José Antônio Camacho to enroll for the PIPE High Tech Entrepreneurial Training program. “The program showed us the importance of developing a business plan for Siprad that was suited for the healthcare market in Brazil,” says Carvalho. To inform their business plan, the two partners visited public and charity-run hospitals in six states.
One of the goals they set for i-Medsys was to cater to demand for TPS systems created by the SUS Radiation Therapy Expansion Plan, under which 140 new linear accelerators would be purchased by the SUS. The initiative was originally launched in 2012 with the Brazilian Ministry of Health’s announcement of a tender procedure for 80 machines, followed six years later by a tender for another 60 machines. Varian was successful in the original bidding procedure and was awarded a contract in 2013 that included delivery of the equipment, construction of manufacturing facilities in Brazil, a training center, and technology transfer.
As of early July, a total of 18 linear accelerators had been delivered, says Izidoro. Under a new agreement concluded in 2018, Varian’s scope of supply increased to 100 machines, in an estimated investment of R$505 million. The remaining 40 machines not under contract and the relevant TPS systems will be purchased under arrangements still to be concluded. It is this gap that i-Medsys hopes to fill.
Invitation to the UN
Siprad is currently being tested at the Radiation Therapy Center (CTR) in Ribeirão Preto and at the Radiology Institute (HC-InRad) in São Paulo. “We’re currently working to improve the target delineation tools and overall program performance. It’s still too early for a definitive assessment,” says radiation oncologist Fábio Prado Luz, of HC-InRad. He believes the program is promising, however, and is developing at a remarkable pace. “The changes we request are promptly implemented, which is something you don’t see in foreign programs. And it makes all the difference.”
Harley Oliveira, who is assessing the Siprad suite at CTR, says the system has a number of key advantages other than only lower costs, including a remote database in the cloud, and the ability to perform planning tasks remotely over the web.
The reduced total cost of ownership and other program differentiators led to an invitation from the United Nations International Atomic Energy Agency’s (IAEA) Division of Human Health to present the project this year at IAEA headquarters in Austria. The agency is seeking software solutions for cancer treatment that can meet demand in developing countries. “We met the agency’s requirements and were encouraged to respond to international tenders once we’ve received required certification,” says Carvalho.
Projects
1. Siprad: Radiation treatment planning systems (RTPS) (nº 15/08412-1); Grant Mechanism Technological Innovation in Small Businesses (PIPE) program; Principal Investigator Diego Fiori de Carvalho (Innolution); Investment R$553,828.32.
2. ArcaMed: A framework for building diagnostic decision support systems (nº 05/60038-5); Grant Mechanism Technological Innovation in Small Businesses (PIPE) program; Principal Investigator Diego Fiori de Carvalho (Innolution); Investment R$471,239.25.