
CNRI / SCIENCE PHOTO LIBRARYTemporary containment: tubercle bacilli engulfed by a macrophage (yellow)CNRI / SCIENCE PHOTO LIBRARY
A freezer set at a very low temperature, minus 70 degrees, holds frozen samples of the microorganism that causes one of the oldest diseases ever recorded in human history, tuberculosis. Locked in a room with restricted access at the State University of Norte Fluminense (UENF), in the city of Campos, Rio de Janeiro State, this freezer contains dozens of tubes of tuberculosis bacteria collected from different regions of Brazil and other countries; some are classified as the most aggressive in spreading the disease in certain regions of the world. Access to this room of UENF‘s biosafety laboratory is strictly controlled by Dr. Elena Lassounskaia, a Russian immunologist. “Only six or seven well trained people are authorized to enter and work in there,” she says. Dr. Lassounskaia was born in St. Petersburg and has worked at UENF for 20 years, where she coordinates studies on the aggressiveness of tuberculosis bacteria.
Experiments done in her laboratory, in collaboration with other teams from São Paulo and Rio de Janeiro, are beginning to generate a new understanding of how the more aggressive strains of tubercle bacilli in some cases are able to overwhelm the immune cells that should be in control. Instead, they spread rapidly throughout the body, resulting in serious damage to the lungs and other organs. In a simplified explanation, published in the July 2014 issue of PLOS Pathogens, the researchers demonstrated that the more aggressive or hypervirulent varieties grow faster in the immune cells of the lungs where they destroy these cells. Chemical compounds signaling to the immune system that cell damage has occurred are also released at an increased rate. In short, these bacteria intensify the release of signals warning of danger, which exacerbates lung inflammation.
In many infections as well as inflammations caused by minor damage, such as a blow to the leg, this alert is healthy and even desirable. It arouses the attention of other immune cells and directs them to the affected area. The new cells that have been summoned surround and digest the dead, infected or damaged cells that sent the chemical alert, thereby solving the problem. In the case of the more persistent microorganisms, such as mycobacteria, which include human and bovine tuberculosis bacilli (Mycobacterium tuberculosis and M. bovis) and leprosy (M. leprae), the immune system has difficulty in completely eliminating the bacteria. It may keep them for years in a restricted area, forming a barrier of immune cells around the infected cells. This collection of cells is known as a granuloma.
When dealing with hypervirulent mycobacteria or patients with compromised immune systems, however, the picture changes. Two years ago a group led by Roland Brosch and Jost Enninga, of the Pasteur Institute in Paris, demonstrated that virulent mycobacteria were able to rupture the sac inside the macrophages which contain the immune cells that detect and surround particles and microorganisms foreign to the body. The rupture of this containment sac known as a phagosome leads to a violent death—necrosis—in which the macrophage explodes and scatters its contents into the surrounding area.
This dramatic death unleashes in the tissue a deluge of signals warning of cell damage. The effect looks like fireworks, indicating danger while attracting more immune cells to the area of the initial damage. But the excess of these warning signals—one in particular, the adenosine triphosphate (ATP) molecule—amplifies the problem: it starts a vicious cycle of infection and macrophage destruction. The São Paulo and Rio de Janeiro researchers demonstrated this in the PLOS Pathogens article (see the infographic).
“In low concentrations in the tissue, ATP acts as the cell activator of the immune system,” says Dr. Maria Regina D’Império Lima, a University of São Paulo (USP) immunologist and one of the coordinators of the study, which includes researchers from the Federal University of São Paulo (Unifesp) and the Federal University of Rio de Janeiro (UFRJ). In this situation, they form narrow channels in the membrane of the immune cells allowing the exchange of ions with the external environment, which thus activates the cells. Already in high concentrations, the ATP induces the appearance of large pores which let out inflammatory molecules, as well as trigger death by necrosis.
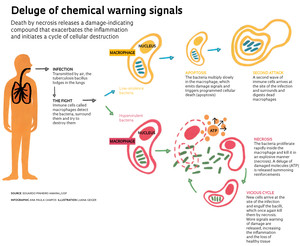 Eduardo Pinheiro Amaral, a biologist and PhD student of Dr. Lima, proved that by killing macrophages through necrosis, hypervirulent mycobacteria strains manage to escape, remain alive and infect more macrophages, leading to exacerbated lung inflammation. While still doing undergraduate research, Amaral developed an infection model in mice to assess the virulence of non-tuberculosis mycobacteria and became one of the first students to be trained to work with mycobacteria in UENF’s level 3 biosafety laboratory. For his Master’s degree, he focused on more aggressive tuberculosis mycobacteria at USP’s biosafety laboratory coordinated by Mario Hirata. Amaral commuted between São Paulo and Campos for about a year to conduct experiments in both laboratories and evaluate a greater variety of bacteria isolated from people and cows with tuberculosis.
Eduardo Pinheiro Amaral, a biologist and PhD student of Dr. Lima, proved that by killing macrophages through necrosis, hypervirulent mycobacteria strains manage to escape, remain alive and infect more macrophages, leading to exacerbated lung inflammation. While still doing undergraduate research, Amaral developed an infection model in mice to assess the virulence of non-tuberculosis mycobacteria and became one of the first students to be trained to work with mycobacteria in UENF’s level 3 biosafety laboratory. For his Master’s degree, he focused on more aggressive tuberculosis mycobacteria at USP’s biosafety laboratory coordinated by Mario Hirata. Amaral commuted between São Paulo and Campos for about a year to conduct experiments in both laboratories and evaluate a greater variety of bacteria isolated from people and cows with tuberculosis.
One group of tuberculosis bacteria studied by Amaral belongs to the Beijing genetic family. “Native to China, this family of mycobacteria is very aggressive and has spread more quickly than others,” says Dr. Lassounskaia. “This is already the predominant strain in Russia and countries of the former Soviet Union and is more resistant to several drugs used to treat tuberculosis.” In Latin America and Mediterranean Europe, the most common strains are from another family of M. tuberculosis, the Latin American-Mediterranean strain, in which cases of drug resistance are relatively rare.
Yet tuberculosis in Brazil and the world remains a challenge for public health. It is estimated that 2 billion people are infected with the tuberculosis bacillus, but in the vast majority of cases, the bacteria remain dormant and only awaken when the immunity level of the body becomes too low, such as in situations of physical or psychological stress or HIV infection. A recent estimate published in the July 22, 2014 issue of The Lancet, shows that the number of people with tuberculosis has increased by nearly 40% in just over 20 years: from 8.5 million in 1990 to 12 million in 2013, although the number of deaths has fallen 12.5% from 1.6 million in 2000 to 1.4 million in 2013. In Brazil, according to this study, there were 108,000 new cases and nearly 6,000 deaths in 2013. “Since 2001, the tuberculosis control program implemented in Brazil seems to have reduced the proportion of new cases from 43 per 100,000 inhabitants in 2001 to the current 36 per 100,000, says Dr. Lassounskaia. “Tuberculosis control is improving, but the problem has not been completely solved.”
Amaral identified the distinct pattern of the immune system’s damage activation signals while working with three varieties of tuberculosis bacteria. He tested two hypervirulent bacteria: the M. tuberculosis Beijing strain isolated from a Russian patient and a strain of M. bovis found in an outbreak of bovine tuberculosis in southern Brazil; although it primarily affects cattle, this variety can cause tuberculosis in humans. The two strains increased more rapidly in immune cells in vitro and in the infected animals than the third bacteria, a strain of M. tuberculosis used as a reference in laboratories.
It took 100 bacteria of the more aggressive strains to initiate a lung infection able to kill. The Beijing strain killed 90% of the rats in eight months, while M. bovis eliminated all the animals in 40 days. The mice infected with the virulent reference strain survived until the end of the experiment. The more aggressive bacteria’s ability to proliferate and the level of inflammation caused (attracting more immune cells) were so high that the lungs of the rodents were left with a mass three to five times higher than normal in just one month. Macrophage death and the recruitment of more immune cells to the infected area destroyed lung tissue and blocked the alveoli, causing shortness of breath and the coughing up of blood.
The role of ATP in this exacerbated inflammation became apparent when the researchers performed the same tests with mice genetically modified not to express the P2X7 protein receptor on the surface of the immune cells; P2X7 recognizes ATP and puts the cells into action. The degree of macrophage necrosis and inflammation in rodents without P2X7 was much lower than in normal ones. “This result answers an important question about the immune response to tubercle bacilli,” says Dr. Lima, who began to notice the importance of this receptor in macrophage death by necrosis in experimental animals with malaria.

CDCMycobacterium tuberculosis secretion of patient with tuberculosisCDC
Besides helping to understand how the more aggressive bacteria act, these findings open up the possibility of more effective intervention in the fight against tuberculosis. “If we can control the inflammation by using compounds to block the activation of the P2X7 receptor, we may be able to gain some important time in treating the sickest patients,” says Amaral. In tests with the more aggressive strains, mice without the protein lived 60-110 days longer than the normal rodents. “Control of the inflammation with compounds acting on P2X7 does not eliminate the bacteria, but it can reduce tissue damage and give antibiotics additional time to act,” he added. Some compounds that act on P2X7 are being tested in animals and humans. If feasible, this complementary strategy could help improve the treatment of the more aggressive forms of tuberculosis, which now takes at least six months and requires the use of four different antibiotics.
Dr. Lassounskaia’s team at UENF and the UFRJ researchers are evaluating the action of some compounds produced by plants on the growth of mycobacteria and the super-inflammation triggered by hypervirulent tuberculosis bacteria. Today traditional anti-inflammatory drugs are used in cases where tuberculosis reaches the central nervous system. But this may not always be the best alternative. “Using anti-inflammatory drugs with unconventional mechanisms of action in combination with antibiotics,” says Dr. Lassounskaia, “seems to be the future of therapy for the more serious cases of tuberculosis.”
Project
Role of inflammasomes in the pathogenesis of tuberculosis caused by hypervirulent clinical isolates of mycobacteria (nº 13/07140-2); Grant mechanism Regular Line of Research Project Award; Principal investigator Maria Regina D’Império Lima – Institute of Biomedical Sciences/USP; Investment R$ 288,936.14 (FAPESP).
Scientific articles
AMARAL, E. P. et al. Pulmonary infection with hypervirulent mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathogens. 3 jul. 2014.
MURRAY, C. J. et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 22 jul. 2014.