For academic researchers, entrepreneurship requires determination, knowledge of the risks, and a good dose of courage to navigate the countless obstacles that will be encountered along the way. So imagine the hurdles to overcome when leaving a well-known field to invest in a more complex and challenging one. This is what the founders of São Paulo–based company Pluricell Biotech decided to do. In 2014, less than a year after it was founded, the biotechnology startup obtained its first funding from FAPESP’s Research for Innovation in Small Businesses (RISB, or PIPE in Portuguese) program for a project aiming to produce human cardiomyocytes. The company’s objective at the time was to offer these cells, which are responsible for making the heart muscle pulsate, to academic researchers to conduct in vitro testing of new drugs.
Almost eight years later, the startup changed course. In April 2021, the company name was changed to LizarBio Therapeutics as it shifted its attention to the international market. It began focusing on the potential of cardiomyocyte grafts for regenerating injured heart tissue in people who have suffered heart attacks. The biotech’s new goal is to develop cell therapies for diseases for which there is currently no cure—a much more challenging objective than offering cells for research into new compounds. The RISB program awarded funding to this new scope in 2021.
This type of change in business direction is known in the corporate world as pivoting. Some people compare it to the pivot of a basketball player who keeps one foot on the ground as a fixed base while rotating their body, searching for the best move to make. The base, in this instance, was the subject biologist Diogo Biagi, one of the founders of Pluricell, studied during his PhD between 2011 and 2014.
To investigate changes in the heart cells of individuals with heart disease, Biagi used their skin cells to generate induced pluripotent stem cells (iPSCs), which are similar to embryonic stem cells, and then transformed them into cardiomyocytes. His PhD, carried out at the Heart Institute (InCor) of the Hospital das Clínicas, School of Medicine, University of São Paulo (USP), was funded by a grant from FAPESP.
The technology for producing iPSCs was developed and published in 2007 by Japanese scientist Shinya Yamanaka, who five years later won the Nobel Prize in Medicine. The technology’s primary application, according to Biagi, has been in modeling diseases and developing new drugs. The cells are obtained from patients and used as experimental models that allow scientists to observe the behavior of diseased cell tissues and their reaction to new compounds.
When Pluricell was founded by Biagi and two colleagues—biologist Marcos Valadares and cardiologist Alexandre Pereira, who is no longer with the company—producing iPSCs for Brazilian laboratories seemed a natural vocation for the business. Biagi’s doctoral supervisor José Eduardo Krieger, director of InCor’s Laboratory of Molecular Genetics and Cardiology, was unsure about the decision. “I found the initial business model strange. They wanted to sell cells, but there was no market. The sector they are working in now—regeneration—is more suitable,” says the physiologist, who was one of the pioneers of stem-cell studies for cardiac regeneration in Brazil in the 2000s.
The partners of the startup, which today numbers 10 researchers, six of whom hold PhDs, soon reached the same conclusion. “We made the decision to pivot at the end of 2017, after concluding that the research market in this field is small and dispersed, even worldwide,” says Valadares, CEO of LizarBio. “We realized there would be more added value in medicine, by offering the product to patients rather than researchers.”
Léo Ramos Chaves
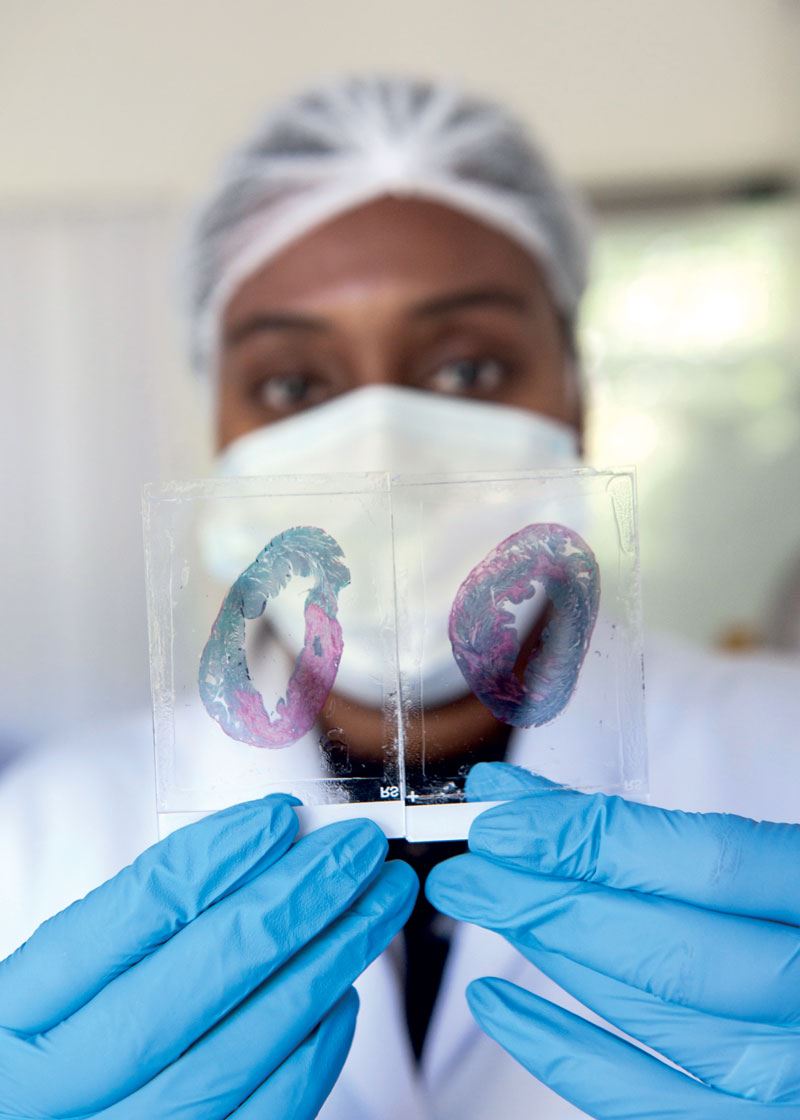
Lab work: a researcher holds up pig heart histological slides…Léo Ramos ChavesWith the change in direction, the startup stopped selling to the medical research market and thus lost its only source of revenue. But it chose to remain at the forefront of the incipient field of regenerative medicine in Brazil, relying solely on investment from funding agencies and the private sector. In 2019, it received US$1 million from pharmaceutical company Libbs Farmacêutica to study regenerative cell therapy for cardiovascular diseases, and in 2021 it was given R$2 million by an undisclosed investment firm. It has also obtained approval from FAPESP for eight RISB projects, totaling roughly R$4.5 million.
The startup’s decision to move into cell therapy for cardiac regeneration was followed by efforts to internationalize. A partnership was formed with Brazilian neuroscientist Alysson Muotri of the University of California, San Diego, who uses pluripotent stem cells in studies of Rett syndrome, a neurological disease caused by a genetic mutation that occurs primarily in girls (see Pesquisa FAPESP issue nº 173).
“Children who suffer from Rett syndrome begin to show signs of developmental delay in the first few years of life. They usually have motor problems and speech and communication difficulties,” says Estela Cruvinel, a biologist and project manager at LizarBio. “It’s a similar spectrum as autism.”
Muotri uses iPSCs to create cerebral organoids: pea-sized, three-dimensional cell models that mimic the behavior of the human brain. With these mini-brains, he has been studying drugs that could treat Rett syndrome, which currently has no cure or specific medication. “I met the Pluricell team some time ago and I was impressed. The connection between us was inevitable and LizarBio was created soon after, incorporating my research on Rett syndrome and other neurological diseases,” says the neuroscientist, who is cofounder of the new company.
With an eye on a potential future treatment for Rett syndrome, the researchers are differentiating iPSCs into glial cells—a varied range of cells found in the brain and other organs of the central nervous system affected by the disease. “Tests using pluripotent stem cells in mice have been started in Alysson’s lab. The first results should be released in the coming months,” says Cruvinel.
Bottlenecks and challenges
Since it was founded, LizarBio has been based at USP’s Center for Innovation, Entrepreneurship, and Technology (CIETEC), but it plans to move to its own headquarters soon. “The aim of the next round of investments is to move into a more adequate space” says Valadares. The new funds will also be used to study how cardiomyocytes can alleviate heart failure, an area of research at a more advanced stage than nerve tissue cell research.
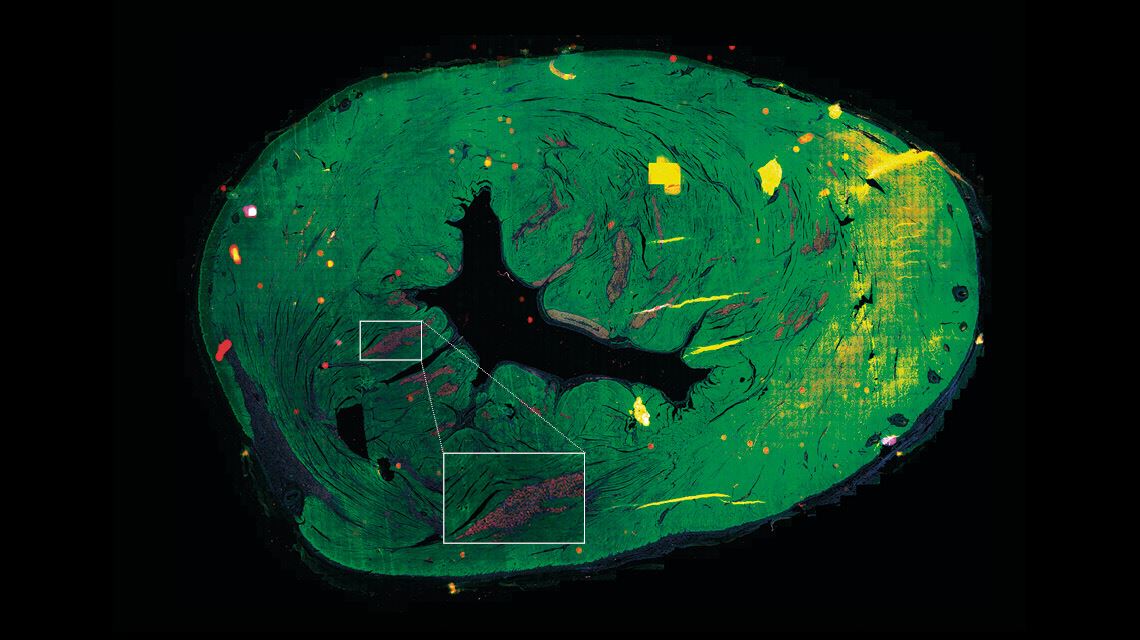
According to Biagi, the RISB phase 2 funding includes the first stage of a study assessing the potential for grafting cardiomyocytes in cardiac tissue, which involves producing and multiplying the cells on a large scale. “The next step, which is an assessment of cardiomyocytes in pigs, still requires further investment, which we hope to obtain soon.” The company has already carried out initial tests to validate the methodology that it will use in preclinical trials involving eight pigs, half of which will receive the stem cells and the other half controls.

Léo Ramos Chaves
…and another looks at human heart cells produced from iPSCsLéo Ramos ChavesTests on rats yielded promising results, described in an article published in the Journal of Personalized Medicine in April 2021. “The injected cells improved cardiac function, although it is impossible to say how many of the cardiomyocytes were retained in the heart,” says Biagi. This is the answer he hopes to obtain from the studies on pigs, whose heart muscles are a similar size to humans’.
According to Krieger, this is one of the biggest bottlenecks in research on cardiac regeneration. “We have successfully developed cardiomyocytes in the lab. The problem now is how to deliver these cells to the heart so that they are incorporated into the organ and function properly.” The physiologist explains that cardiomyocytes made from stem cells are still immature cells. While they are better at proliferating—something that is necessary for cardiac tissue regeneration—there is also a greater risk that they will be rejected or cause arrhythmias. “Cardiac function is rhythmic; the cells need to work together,” explains Krieger.
Another challenge is the number of cells that need to be produced for the experimental treatment. While the group injected around 10 million cardiomyocytes into rats, the pig trial will require around 1 billion cells per animal. This number is similar to how many cells a human loses when they suffer a heart attack. The optimal number of cells to graft into the heart tissue is still being investigated at LizarBio.
To increase its production capacity, the company entered into a partnership with the São Paulo State Institute for Technological Research (IPT) via USP’s Inter-unit Graduate Program in Biotechnology. Under the supervision of IPT researcher Patrícia Leo, LizarBio’s Sirlene Rodrigues is studying the cultivation, multiplication, and differentiation of iPSCs into cardiomyocytes as part of her master’s degree, with funding from FAPESP. The goal of the partnership with the IPT is to master mass production, which would reduce the cost of producing cardiomyocytes.
Another obstacle to regenerative medicine is current regulations on the use of stem cells, but in this regard, Brazil has made an important advance in recent years, highlights Valadares. “Until 2018, there was no formal regulatory framework allowing for the registration of advanced therapeutic products,” he says. “In February 2020, Brazil’s Health Regulatory Agency [ANVISA] published RDC [Collegiate Board Resolution] 338, which governs the registration of these products.”
In Japan, the government authorized clinical trials using iPSCs in 2013. Several research groups from other countries continue to look for an effective cell therapy. “Encouraging results are attracting more funding from companies. When the risk decreases, private capital input increases,” says Krieger.
There is still no guarantee that regenerative therapy will be available in the short term, says Marimelia Aparecida Porcionatto, a professor of molecular biology at the Federal University of São Paulo (UNIFESP) and a researcher at the National Institute of Science and Technology for Regenerative Medicine (INCT-Regenera), a research network composed of 28 associated laboratories from different institutions. “The entire development process can take 10 to 20 years—it’s hard to predict,” she says. “There has been a lot of progress since the 2000s, when labs began more actively studying mesenchymal stem cells isolated from adult tissue. It was thought that they would fix all evils, but they didn’t.”
Mesenchymal stem cells have not proven effective in cardiac regeneration, for example. “Today, we know that they play a more immunomodulatory role, reducing local inflammation,” explains Porcionatto. They could still play an important role in repairing diseased hearts, however. Krieger agrees: “It is a new technique with great potential for improvements, but it is not a miracle cure.”
With much hope now placed on induced pluripotent stem cells, the researchers at LizarBio know that in addition to technical, economic, and legal challenges, they also have to deal with high expectations. “We know how long things can take and we are carefully planning each step,” concludes Valadares.
Projects
1. Assessment of the potential of iPSC-derived human heart cell transplantation in pigs (nº 21/01413-3); Grant Mechanism Research for Innovation in Small Businesses (RISB); Principal Investigator Estela Mitie Cruvinel (Pluricell); Investment R$799,480.55.
2. Human heart cell generation for use in cell therapy (nº 18/22552-9); Grant Mechanism Research for Innovation in Small Businesses (RISB); Principal Investigator Diogo Gonçalves Biagi dos Santos (Pluricell); Investment R$92,872.20.
3. Commercial feasibility of induced pluripotent stem cell-derived keratinocytes and skin equivalent development (nº 16/50082-1); Grant Mechanism Research for Innovation in Small Businesses (RISB); Agreement FINEP – RISB/PAPPE; Principal Investigator Estela Mitie Cruvinel (Pluricell); Investment R$619,024.72.
4. Characterization of induced pluripotent stem cell-derived cardiomyocytes and standardization of cellular assays (nº 15/50224-8); Grant Mechanism Research for Innovation in Small Businesses (RISB); Principal Investigator Diogo Gonçalves Biagi dos Santos (Pluricell); Investment R$1,020,338.16.
5. Generation of induced pluripotent stem cell-derived fibroblasts and keratinocytes and their characterization for use in drug trials (nº 14/50225-1); Grant Mechanism Research for Innovation in Small Businesses (RISB); Principal Investigator Estela Mitie Cruvinel (Pluricell); Investment R$637,535.68.
6. Differentiation of induced pluripotent stem cells into hepatocytes and their characterization for use in drug trials (nº 13/50263-8); Grant Mechanism Research for Innovation in Small Businesses (RISB); Principal Investigator Marcos Costa Valadares (Pluricell); Investment R$183,058.49.
7. Standardization of a human cardiomyocyte cell platform for in vitro drug testing (nº 13/50076-3); Grant Mechanism Research for Innovation in Small Businesses (RISB); Principal Investigator Diogo Gonçalves Biagi dos Santos (Pluricell); Investment R$1,038,779.60.
8. Using iPS (induced pluripotent stem) cells to understand changes in cardiomyocytes from patients with genetically inherited cardiomyopathies (nº 10/13426-8); Grant Mechanism Doctoral (PhD) Fellowship; Supervisor Diogo Gonçalves Biagi dos Santos (Pluricell); Investment R$118,557.73.
9. Biotechnological production and in vitro characterization of cardiomyocytes in anchorage-independent systems from induced pluripotent stem cells for cell therapy (nº 20/06673-0); Grant Mechanism Master’s (MSc) Fellowship; Supervisor Patrícia Leo (IPT); Investment R$36,509.88.
Scientific article
BIAGI, D. et al. In situ maturated early-stage human-induced pluripotent stem cell-derived cardiomyocytes improve cardiac function by enhancing segmental contraction in infarcted rats. Journal of Personalized Medicine. May 4, 2021.