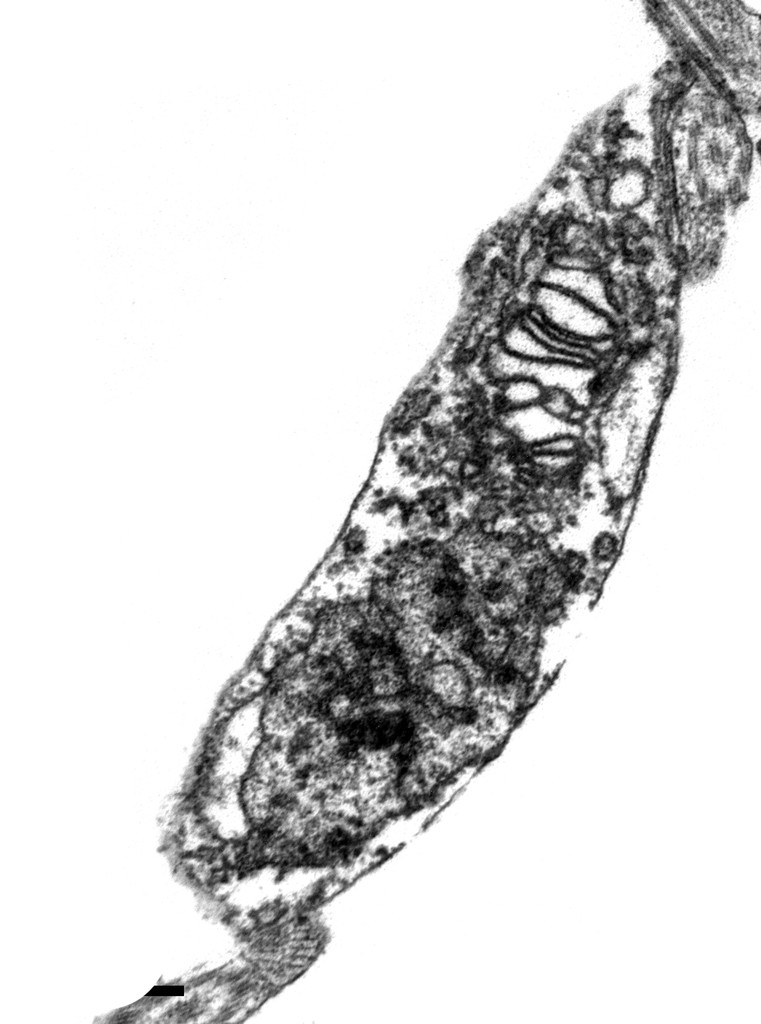
André Tempone / Adolfo Lutz Institute
In a transmission electron microscope, two snapshots of Trypanosoma cruzi under the effect of the drug sertraline: after 30 minutes…André Tempone / Adolfo Lutz InstituteTreatment for two infectious diseases, Chagas disease and Leishmaniasis, is gaining new ground with drugs formulated by Brazilian researchers. The World Health Organization (WHO) classifies these diseases as neglected. As they mainly affect low-income populations of tropical regions of the planet, the pharmaceutical industry does not invest heavily in developing and marketing drugs to treat them. As a result, combating these diseases may involve developing molecules with new pharmaceutical formulations, drug combinations and also less toxic forms for use of existing drugs.
Chagas disease, caused by the protozoan Trypanosoma cruzi, is transmitted by the kissing bug and, according to WHO estimates, affects 8 million people worldwide, with 12,000 deaths annually and 65 million people at risk of exposure. Leishmaniasis, transmitted by bloodsucking insects of the order Diptera, commonly known as sand flies, has two manifestations: visceral, which can be fatal, and cutaneous. WHO estimates there are 12 million people infected worldwide, with 30,000 deaths per year and 350 million people living in at-risk areas.
One potential new drug candidate was identified by researchers Wagner Vilegas, of the Institute of Biosciences of São Paulo State University (Unesp) in São Vicente, Emerson Ferreira Queiroz, of the University of Geneva, Switzerland, and Cláudia Quintino da Rocha, of the Federal University of Maranhão (UFMA) in northeastern Brazil. They isolated a new family of molecules from a plant found in the Cerrado savannah, Arrabidaea brachypoda, known as beer-of-the field and used to treat kidney stones. Based on in vitro and in vivo results of studies in laboratory animals, the researchers believe that a molecule of the family has the potential to be developed as a new drug. “An important fact is that the substance showed no toxicity at the doses tested,” says Rocha. Benznidazole, the drug most often used today in Brazil to treat Chagas disease, has severe side effects, such as allergic skin reactions, nausea and vomiting.
Rocha says the newly tested molecule can be produced in the laboratory, because the synthetic route was developed during her postdoctoral training with the group at the University of Geneva. The team has also filed a patent application in Brazil, to be extended internationally. For now, the researchers have the support of FAPESP and the NGO Drugs for Neglected Diseases (DNDi), a reference institution supporting research in this area. The team is still looking for partners in the pharmaceutical industry to enable clinical testing in humans.
Professor José Rodrigues Coura, head of the Parasitic Diseases Laboratory of the Oswaldo Cruz Foundation (Fiocruz), located in Rio de Janeiro, says that the researchers’ initiative is important given the limitations of existing drugs. “We do not have an ideal drug to treat Chagas disease. Several empirical trials with antimalarials, antibiotics and more than 30 drugs did not yield promising results,” he says.
As explained by Coura, the first effective drug arrived just 50 years after the discovery of Chagas disease, in 1909, when Zigman Brener, of the Federal University of Minas Gerais (UFMG), located in Belo Horizonte, showed that nitrofuran furacin cured 95% of mice with Trypanosoma cruzi. However, this drug produced severe polyneuropathy (a neurological disorder) in treated patients and its use was prohibited. Nitrofuran improvements led to the drug nifurtimox. Benznidazole emerged sometime later. The two substances, according to Coura, can cure 70% to 80% of acute cases and 20% of recent or chronic cases, but for cases requiring long 60-day treatments, there are significant toxic reactions that force cessation of 10% of these treatments. Coura believes the research with Arrabidaea brachypoda will now enter a decisive phase to see if it really works. “Of every 2,000 promising drugs, only one turns into a product suitable for human treatment,” he says.
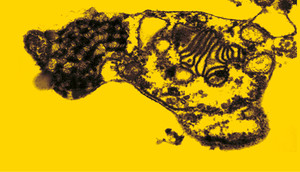
André Tempone / Adolfo Lutz Institute
… and after one hour of treatmentAndré Tempone / Adolfo Lutz InstituteRepositioning drugs
André Tempone, a researcher at the Adolfo Lutz Institute, is pursuing another line of research, with multidisciplinary teams working on the repositioning of existing drugs and combination therapies. An advantage of this approach, according to Tempone, is that it reduces costs and the amount of research time, because the drug has already been tested for toxicity. He is currently studying the therapeutic potential of oral antidepressants based on sertraline for their use in visceral and cutaneous leishmaniasis and Chagas disease.
“We are continuously screening existing drugs already on the market. We select a candidate for repositioning when the concentration that kills the parasite is below 10 micromolars [measurement equivalent to one millionth of a mole, a unit used to measure the concentration of molecules],” says Tempone. The work indicated that sertraline was potent in vitro in cells against Leishmania infantum, an agent of a fatal form of the disease in Brazil, visceral leishmaniasis. The same was true for Leishmania amazonensis, one of the species that causes cutaneous leishmaniasis in Brazil. “And we found this activity was also true for Trypanosoma cruzi, where sertraline killed the parasite and preserved the host cell.”
Since in vitro results often cannot be repeated in vivo, animal testing has already begun and is being conducted by the Adolfo Lutz Institute, the universities of Dundee, Scotland, San Pablo, Spain, and by the School of Pharmaceutical Sciences of the University of São Paulo (FCF-USP), with support from FAPESP. If the results are positive, the next step, says Tempone, will be to combine sertraline and benznidazole to treat Chagas disease. “The combination can extend efficacy, reduce toxicity and even lower the parasite’s resistance,” he says. Initial preclinical studies have indicated activity in an animal model of visceral leishmaniasis and acute Chagas disease, reducing the number of parasites. “We hope that, using different doses based on studies in progress, we can develop a therapy that eliminates over 95% of the parasites in the animal model,” says Tempone.
To treat leishmaniasis, sertraline will be tested in combination with the drugs amphotericin B and miltefosine, since this second drug is still being used in a clinical study in Brazil. In Brazil, the drug most widely used to treat leishmaniasis is an antimony compound; its use for this purpose was discovered by Gaspar Vianna, a researcher from Pará State, in 1912. Antimony, says Tempone, treats the patient, but does not eliminate 100% of the parasite, and it still has serious adverse side effects, especially for patients with cardiac and renal problems.
Tempone is also using Brazilian biodiversity as a source of research for the development, though still early, of two bioactive molecules for prototype drugs to treat the two tropical diseases. One such effort is in partnership with Roberto Berlinck, of USP São Carlos, which develops molecules from marine organisms. The other molecule is being developed with João Lago, of the Federal University of São Paulo (Unifesp), and is based on a plant of the Cerrado savannah, Nectandra leucantha, known as cinnamon-dry or cinnamon-white.
Nanoparticles on the skin
Treating cutaneous leishmaniasis is the focus of researcher Bartira Rossi-Bergmann’s work at the Federal University of Rio de Janeiro (UFRJ). Although it is not fatal, the disease does have serious social consequences for the 1.2 million people infected worldwide each year. Today the fight against this disease is done with daily injections of antimony. These injections are available only at health centers and hospitals. In addition to severe side effects, the difficulty of access to health centers, particularly by residents of isolated communities, causes numerous patients to discontinue treatment. Rossi-Bergmann’s work envisions a drug given in a single dose by means of an implant of nanoparticles at the infected site, with the gradual release of the drug into the skin.
She is studying two drugs as possibilities, which may, in the future, be combined into a single implant. The most promising synthesizes a molecule identified from the plant, Piper aduncum, a member of the pepper family. The CH8 chalcone molecule, a joint patent application of UFRJ and the Federal University of Santa Catarina (UFSC), has lipophilic characteristics (soluble lipids) which makes it easier to encapsulate. But because it is a new molecule, it still has to be tested in animals and humans. The study is being supported by the Brazilian Innovation Agency (FINEP).
Rossi-Bergmann is also counting on a line of development that would win faster approval from the Brazilian Health Surveillance Agency (Anvisa). For this she will use the amphotericin B drug, which is already used to treat Leishmaniasis, although it is difficult to encapsulate. She says that tests conducted with mice indicate that an amphotericin B implant in the skin does not generate the side effects common to the drug when it is injected into muscle. Both the chalcone and amphotericin B particles are protected with patent filings. The particles will now be synthesized on a larger scale according to the standards of good manufacturing practices (GMP) for the production of a certified pilot batch. This will be followed by pre-clinical and clinical trials in humans. “Now, we need financial support to continue,” says Rossi-Bergmann. We estimate an investment of R$7 million until completion of Phase 1 of the pre-clinical tests. “We’re talking to research-sponsoring agencies and also pharmaceutical companies such as Biolab, GlaxoSmithKline and GC-2,” she says. Her team also created a startup company, LeishNano, in order to attract investors to the project.
Projects
1. Standardized herbal medicines for the treatment of chronic diseases (nº 2009/52237-9); Grant Mechanism Biota Program – Thematic Project; Principal Investigator Wagner Vilegas (Unesp); Investment R$ 1,805,600.07 and US$ 1,163,945.04.
2. Rational pre-clinical study of new drug candidates against neglected protozoan infections using pharmacokinetic approaches (nº 2015/23403-9); Grant Mechanism Regular Research Grant; Principal Investigator André Gustavo Tempone Cardoso (Adolfo Lutz Institute); Investment R$147,545.00.