
Plymouth University
In 2015, chemist Roy Lowry (left) reproduced the cannon at the University of PlymouthPlymouth UniversityFor a number of years, videos showing an intriguing experiment, which has now received a satisfactory physical explanation, have been circulating the internet. In the video, a plastic bottle with a small amount of liquid nitrogen transforms a barrel full of ping-pong balls into a flashy cannon. While a liquid at temperatures below -196.15 degrees Celsius, nitrogen quickly transforms into gas at room temperature, undergoing an explosive expansion that shoots thousands of balls into the air. The experiment has already been done on TV shows and briefly created a scene in a campus building at Plymouth University in England, when chemist Roy Lowry performed it in 2015 (bit.ly/1PabSI6). The curious thing is that at the moment of the explosion, it is not only the balls that go flying. The barrel also comes off the ground, sometimes rising as high as one meter, whereas it was expected that the barrel would remain stationary or ricochet.This apparently simple experiment has been explained by Brazilian and German researchers. In an article published in April in the American Journal of Physics, physicists Jason Gallas, from Rio Grande do Sul, and Eric Parteli, from Pernambuco, along with Santa Catarina engineer Daniel Nasato, in partnership with Germans Thorsten Pöschel and Patric Müller, showed that it is the friction between the balls themselves and between the balls and the barrel that makes the barrel fly.
Various attempts at explaining this experiment had already arisen on internet discussion forums. Some suggested that the explosion caused the bottom of the barrel to deform and press into the ground, which in turn would push the barrel upwards. Others proposed that the group of balls, when ejected, formed a partial vacuum that dragged the barrel upwards. The third explanation was more complicated. The nitrogen bottle was floating in water. The blast threw the balls up while pressing the water against the bottom and walls of the tub. As it retreated, the water would then pull the barrel up, making it jump.
These interpretations would explain the barrel’s rise under certain conditions but, according to the researchers, were not relevant to the phenomenon observed. The barrel took off even when it was made of metal and had a rigid bottom. The group also saw no reason why the explosion would create a vacuum since the displaced air was quickly filled with nitrogen. If the vacuum was relevant, then the barrel would still come off the ground even without the balls present, which was not the case. The third explanation, to be valid, would require that the barrel be slightly off the ground initially or that the floor be of an elastic material, whereas in many experiments, the barrel was on a hard, dry floor. “We were looking for a general explanation,” says Nasato, now a researcher at the Technical University of Munich, Germany.
The group started thinking about this experiment in 2011, when Gallas first saw the video. He arrived at the University of Erlangen-Nuremberg, Germany, for a term as a visiting professor and brought the subject up in an informal discussion of physics subjects, the “kaffee seminar.” Thorsten Pöschel, the director of the university’s Institute for Multiscale Simulation, found the problem interesting. Similar to Gallas, he specializes in the behavior of granular materials (grains of sand, food, and ores) and suspected that the barrel would bounce as a result of a known effect in his field: clogging.
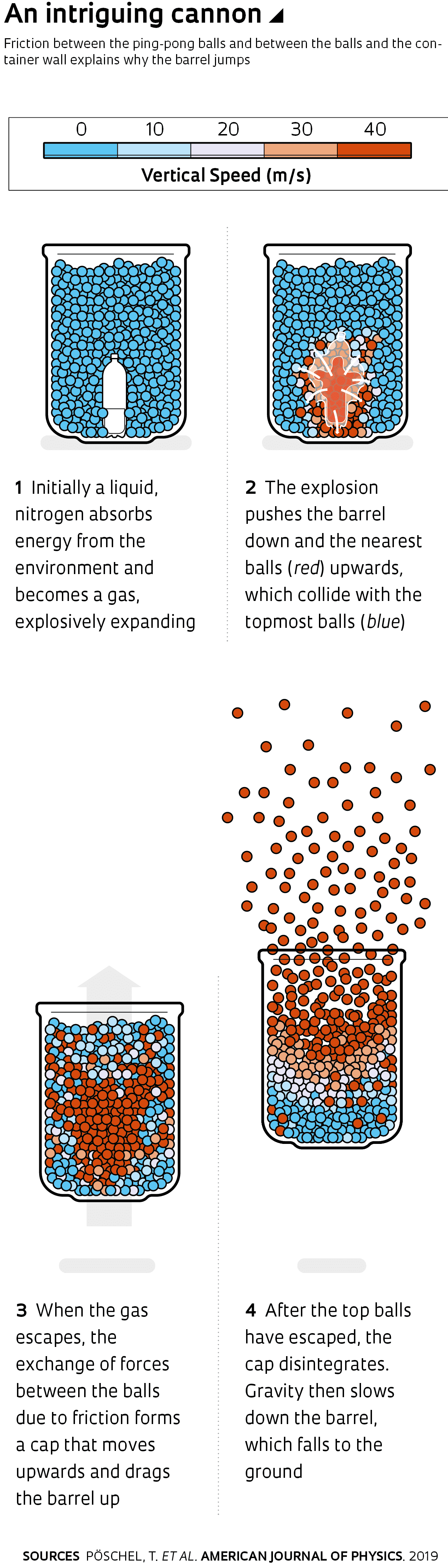
Since it was not possible to measure all the forces at work in the experiment, they turned to computational simulations. Parteli, now at the University of Cologne, Germany, performed the first simulations, confirming their initial supposition. However, simulations that duplicated the parameters of the Plymouth experiment (the number of balls, along with their dimensions and properties, as well as those of the barrel) had to wait until Nasato’s arrival at Erlangen for a postdoctoral fellowship. Nasato then obtained realistic results that correctly reproduced the height reached by the barrel.
At the moment of the explosion, the balls nearest the nitrogen bottle are ejected at high speed and collide with the balls in the upper layers, which are pushed upwards and clog the passageway. The exchange of forces between the balls due to friction forms what physicists call chains of force and causes the balls to behave, for a fraction of a second, as a single rigid object—like a large cork—moving at high speed. The friction between the cap formed by balls and the barrel wall drags the barrel upwards until the upper balls escape and let the other balls also pass.
However, this by itself does not explain the barrel’s upward movement, which also occurs because the acceleration of the balls moving upwards and the exchange of forces between the balls and the barrel wall occur at different moments. Immediately after the explosion, the expanding nitrogen simultaneously and equally pushes the balls upwards and the bottom of the barrel downwards, which would normally keep the barrel on the ground. However, after the gas escapes and stops exerting its force, the balls forming the cap continue to move upwards due to inertia. It is at this point that the friction between the balls and the barrel wall drags the barrel upwards. After rising for a short time, gravity slows the barrel down, which then falls to the ground.For Allbens Atman, a physicist at the Federal Center for Technological Education of Minas Gerais (CEFET-MG) and a specialist in granular media, the explanation is surprising for two reasons. First, the system formed by the barrel, balls, and nitrogen bottle is not isolated from the environment, as some might think. “The surrounding ambient temperature, which is well above that of liquid nitrogen, provides the energy that generates the explosion,” he explains. “This negates the apparent paradox, which at first leads us to wonder where the force that lifts the barrel comes from.” Second, friction is necessary and sufficient to drag the barrel upwards. In the simulations, after removing the friction between the balls or between them and the barrel wall, the barrel does not leave the floor.
“Understanding the role of friction in the interaction of granular materials can lead to industrial applications,” Nasato observes. In the pharmaceutical field, it could help design better pneumatic conveyance systems (pressurized airflow in tubes) for materials that must be kept sterile, reducing the risk of clogging and equipment breakage. It can also assist in the calculation of optimum feed speeds for ore refinery blast furnaces and in the production of ultrafine powders used for producing new materials.
Scientific article
PÖSCHEL, T. et al. Ping-pong ball cannon: Why do barrel and balls fly in the same direction? American Journal of Physics. v. 87, i. 4, p. 255–63. April, 2019.
Published in July 2019
Republish