In its April 25 edition, American newspaper The Washington Post published a worrying article. Doctors from hospitals in New York, Baltimore, and Philadelphia have reported an increase in an unusual phenomenon: the occurrence of major strokes in relatively young adults, almost always in people with none of the risk factors, which include high blood pressure, smoking, excessive alcohol consumption, and obesity. The common characteristic among the patients is that they had been infected with the novel coronavirus, SARS-CoV-2, which causes COVID-19, although not all of them were symptomatic. “We are used to thinking of 60 as a young patient when it comes to large vessel occlusions [bloodclots in the brain],” Eytan Raz, assistant professor of neuroradiology at New York University, told the newspaper. “We have never seen so many in their 50s, 40s, and late 30s,” added the doctor, coauthor of a paper submitted for publication that describes the findings.
The cases involve blockages of vessels that supply blood to the brain, known as ischemic strokes, and are still low in number—with just a few dozen recorded in the USA. The first were reported several weeks before the Washington Post article, by doctors in Wuhan, the Chinese city where the virus first arose in late 2019. But there, the neurological problem affected older and severely ill people, to the extent that it initially took some time for a connection to be identified between strokes and SARS-CoV-2. At that time, there were just a few thousand cases of COVID-19, and deaths were still in the hundreds, all in China. As the virus rapidly spread, less common but equally serious consequences of the disease began to come to light.
Eight out of 10 people who contract the virus are asymptomatic or suffer only moderate symptoms, the most frequent of which are fever, a dry cough, and fatigue, which occur gradually, about a week after the individual contracts the infection. Some also experience general aches and pains, a sore throat, and nasal congestion. As the epidemic (an outbreak restricted to one or a few countries) became a pandemic (global in scale), other symptoms became associated with the disease, such as diarrhea and loss or reduction of smell and taste, in addition to numbness in the hands and feet, dizziness, confusion, delirium, seizures, and ischemia. In 20% of those infected, COVID-19 manifests as a serious illness, causing high fever, severe cough, and shortness of breath, all signs of pneumonia.
– Science fights back
– The antivirus arsenal
– Progress toward dengue vaccine
– Vital ventilators
– In the palm of the hand
– Michel Nussenzweig: Antibody hunter
– Historical vulnerability
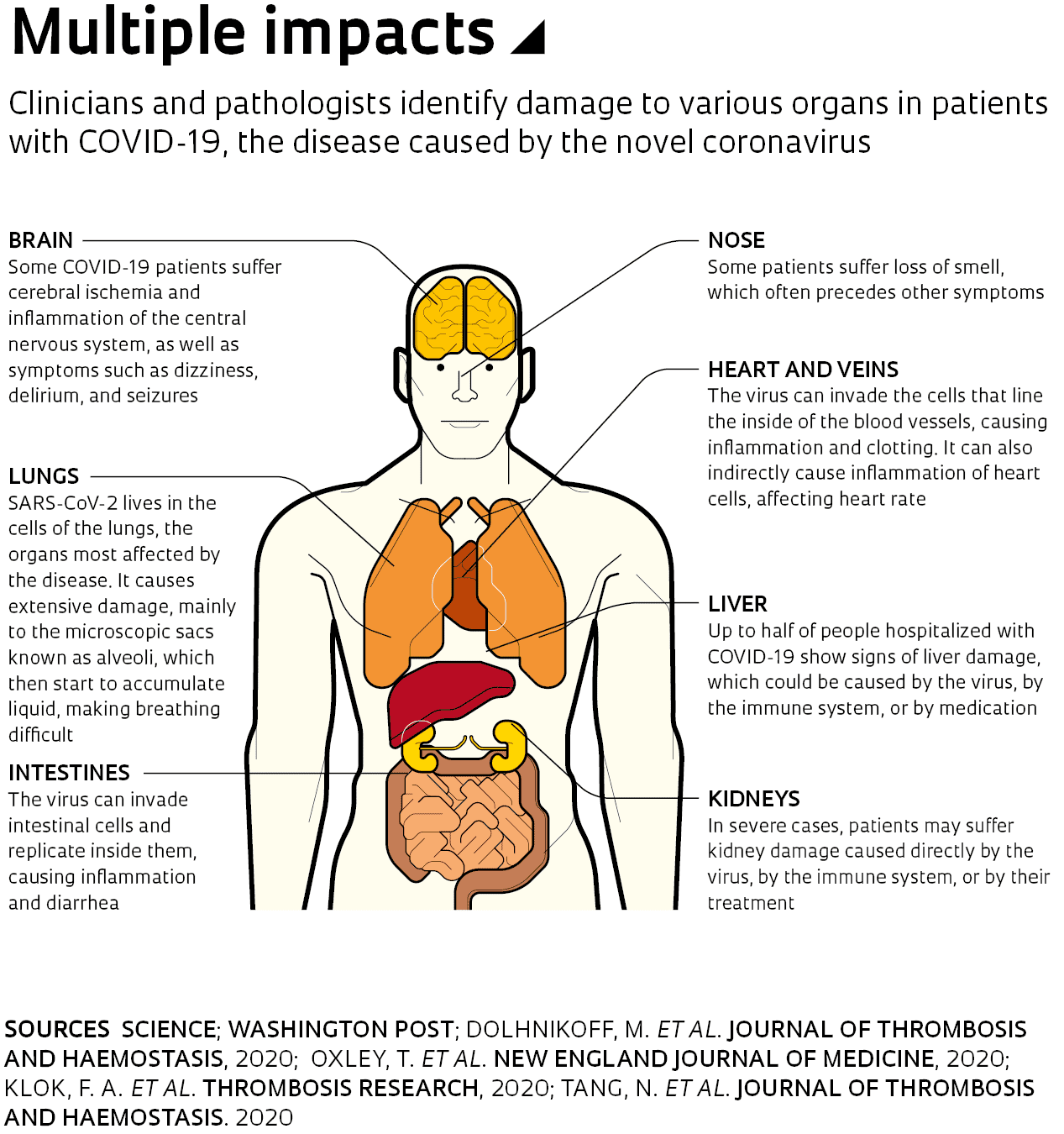
Until a few weeks ago, doctors, government officials, and the media were focused on the increasing number of cases and on those with more serious symptoms requiring hospitalization, who could overload health systems in countries severely affected by the pandemic. As the virus advances and studies attempt to understand how it behaves, medical reports and scientific articles have begun to suggest that the damage caused by the infection may go far beyond the lungs. SARS-CoV-2 can indirectly affect the heart, kidneys, intestines, central nervous system, and blood vessels. “The disease can attack almost anything in the body with devastating consequences,” said Harlan Krumholz, a cardiologist at Yale University who investigates critical COVID-19 cases, in a statement to the journal Science on April 17. “Its ferocity is breathtaking and humbling.”
Initially, COVID-19 was described as pneumonia of unknown origin that caused more severe damage to the lungs than experts were used to seeing. Transmitted through the air or by physical contact with infected surfaces, the virus begins its journey in the human body by attaching itself to the layer of cells that lines the nose and throat. There it multiplies, and if the immune system is not able to fight it off, it begins to spread through the airways until it reaches the lungs. In these respiratory organs, SARS-CoV-2 causes extensive and severe lesions, mainly affecting the alveoli—microscopic air sacs where oxygen and carbon dioxide are exchanged. In the worst cases, patients can deteriorate quickly, leading to severe acute respiratory syndrome. Breathing becomes very difficult and blood oxygenation levels drop, impairing the functioning of other organs.
“The damage caused by the new coronavirus is very serious,” reports pathologist Marisa Dolhnikoff, head of a team from the University of São Paulo’s School of Medicine (FM-USP) that is performing autopsies on people who have died from COVID-19. “In patients who develop the most aggressive form of the disease, the lesions are very similar to those that occur in Severe Acute Respiratory Syndrome [SARS] and Middle East Respiratory Syndrome [MERS], both caused by other types of coronavirus.”
Since the first COVID-19 death was recorded in Brazil on March 17, the FM-USP group has performed minimally invasive autopsies on 20 bodies. Of these, the results of 10 have already been analyzed—five men and five women, with ages ranging from 33 to 83 years. Eight of the victims were over 60 years old and seven had chronic diseases (diabetes, hypertension, and heart problems). In the initial analysis, pathologists found lesions caused by the virus throughout the respiratory system, with the most severe in the alveoli.
Artyom Geodakyan / TASS / SIPA USA / Fotoarena
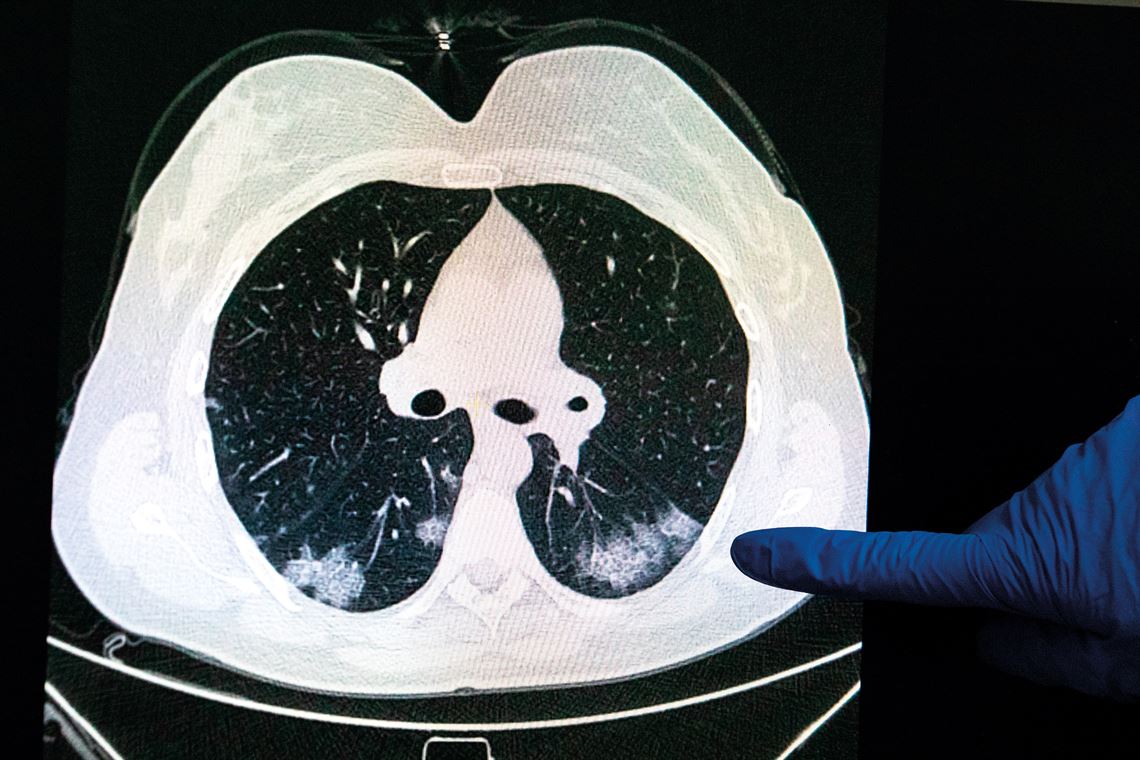
In Moscow, Russia, a doctor examines a CT image of a person’s lungs, showing typical COVID-19 patchesArtyom Geodakyan / TASS / SIPA USA / FotoarenaComputed tomography (CT) scans of the lungs showed multiple white patches with ground glass opacity. Characteristic of some forms of pneumonia, these patches covered the entire posterior border of the two respiratory organs. These areas were full of damaged pneumocytes (epithelial cells that cover the inside surface of the alveoli). The infected cells were sometimes larger than usual, as was their nucleus, where the genetic material (DNA) is stored. This is typical of a viral infection, causing the surface layers of the alveoli to flake off. “Major epithelial lesions are characteristic of COVID-19,” says Dolhnikoff. Without their internal cell lining, the alveoli start to accumulate liquid secreted from the surrounding blood capillaries. “This liquid hinders the exchange of gases and leads to respiratory failure,” says Amaro Duarte Neto, a pathologist on the USP team.
Previous studies have already indicated that SARS-CoV-2 has an affinity for the cells that line the surfaces of the respiratory system. The cells that comprise the mucous membrane, known as epithelial cells, contain a protein—angiotensin-converting enzyme 2 (ACE2)—that facilitates entry of the virus. In an article published on the medRxiv repository on March 27, the group, led by USP biologist Helder Nakaya, found that expression of the gene responsible for producing ACE2 was greater in the lungs of people with hypertension, diabetes, and chronic obstructive pulmonary disease than those who did not have these health problems. The results could help scientists to understand why people with these diseases are more at risk of suffering more severe symptoms of COVID-19.
Some researchers also suspect that the high concentration of ACE2 in the nasal mucosa allows its cells to act as an entry point for the virus into the central nervous system. A past study on rodents showed that SARS-CoV, the coronavirus that causes SARS and is related to SARS-CoV-2, is able to penetrate brain tissue via neurons at the back of the nose that connect to the region of the brain responsible for odor detection. If this also occurs with SARS-CoV-2, it could explain a symptom recently recognized as typical of COVID-19: the loss of smell (anosmia) and taste (ageusia). A survey of 417 people with the disease in Belgium, France, Italy, and Spain, published in the journal European Archives of Oto-Rhino-Laryngology in April, found that at least 86% of respondents suffered anosmia before the onset of other symptoms, even if their nose was not blocked.
The fact that the virus can affect the central nervous system could also explain why some people may develop neurological complications, such as dizziness, confusion, delirium, and seizures, in addition to ischemia, all of which are relatively frequent in severe cases of COVID-19. “Current evidence remains scarce and additional work is needed on whether neurological manifestations occur in COVID-19 patient populations beyond those of the initial studies,” wrote neuroscientist Fernanda de Felice of the Federal University of Rio de Janeiro (UFRJ) and her colleagues in a review paper published in the journal Trends in Neurosciences on April 21. If these neurological symptoms are common, experts will have to investigate the underlying cause. The data available so far do not indicate whether they are a result of direct damage caused by the virus or an excessive response by the body’s own immune system.
In the autopsies performed at USP, the researchers also found evidence of another phenomenon that is being seen more and more in serious cases of the disease: excessive formation of blood clots (thrombi). In eight of the 10 cases studied, the small arteries of the alveoli contained microthrombi, in addition to small hemorrhages. These phenomena have opposite causes—the former arise from excessive blood clotting and the latter from insufficient clotting—and indicate that the people who died of COVID-19 had disorders associated with blood flow. “These findings support the current theory that critically ill patients are suffering from hypercoagulability,” wrote Dolhnikoff and her colleagues in an article on the results of the autopsies, published in the Journal of Thrombosis and Haemostasis on April 15.