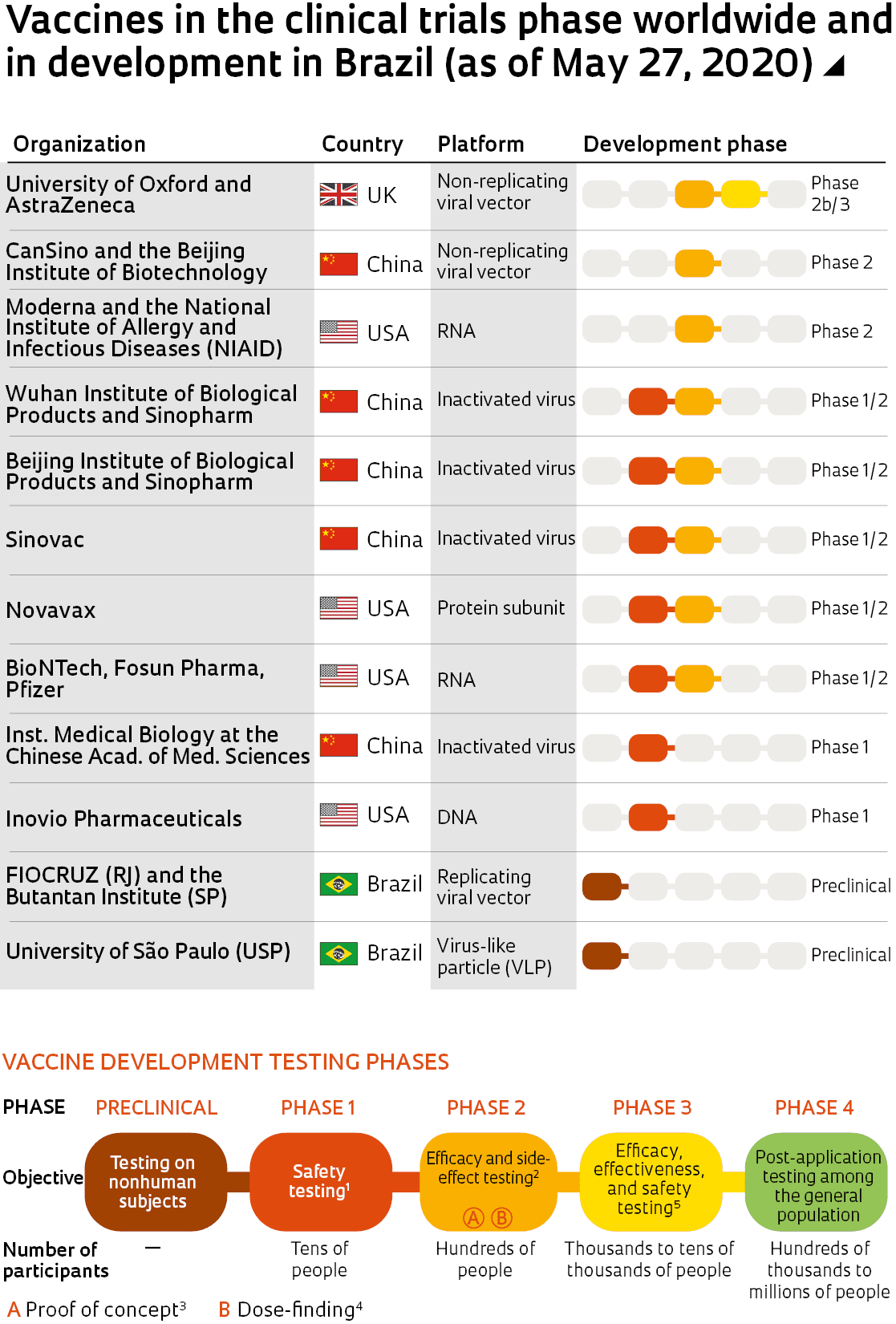
Notes (1) Testing the effects of the product to identify adverse reactions (2) Whether the expected effect is achieved under controlled conditions (3) Demonstration that validates a certain theory (4) dose which produces the desired effects without causing harmful side effects (5) Ability to achieve the expected effect on a large scale under real-world conditions
Sources World Health Organization, Draft landscape of Covid-19 candidate vaccines – May 27, 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. Phases of vaccine development: https://www.ncbi.nlm.nih.gov/books/NBK236428/
Republish