Eurico de Arruda Neto is a student of viruses, organisms on the edge between what is living and non-living and that are so lean they depend completely on other living organisms to reproduce and evolve. “They are extremely elegant biochemical parasites,” confirms the virologist, who during his undergraduate at the Federal University of Ceará (UFC), became interested in these microscopic beings, which are basically comprised of genetic material surrounded by a layer of proteins.
In the last years of his medical studies, he participated in a project with North American researchers who were investigating the most common infections in a Fortaleza slum and discovered the importance of respiratory viruses to human health which, to that point, had received little attention. During his master’s at the University of São Paulo (UNIFESP), he studied the occurrence of HIV among Brazilian indigenous populations, and during his doctoral studies, which he began in the same institution and completed at the University of Virginia, USA, he returned to the study of respiratory viruses. While there, at the end of the 1980s, he had his first contact with coronaviruses, which at that time were already known for infecting humans and causing respiratory problems similar to flus or colds.
As he went back and forth, these viruses, which are responsible for about 10% of respiratory problems in adults and children, did not leave his radar. Covid-19, caused by SARS-CoV-2, is the most recent member of the coronavirus family and its pandemic has caused Arruda to reorganize the work routines of his research group to investigate how the new pathogen acts in the human body and to seek ways to fight it.
In the following interview, given at the beginning of January by video conference, the virologist and coordinator of the Viral Pathogenesis Laboratory at the Faculty of Medicine at the University of São Paulo in Ribeirão Preto (FMRP-USP), speaks about the surprising capacity of SARS-CoV-2 to spread, the emergence of new variants, and the risk they present to the efficacy of some types of Covid-19 vaccines. He also advises that more up-to-date surveillance systems be created to detect viruses that can cause new pandemics.
Field of expertise
Virology
Institution
University of São Paulo (USP)
Educational background
Bachelor’s degree in Medicine from the Federal University of Ceará (1982), Master’s in Infectology from the University of São Paulo (1987) and PhD from the same institution, with an exchange to the University of Virginia (1991)
Published works
83 scientific articles and 20 book chapters
The coronaviruses are your old acquaintances. When did you begin to study them?
I decided to study respiratory viruses soon after I finished my undergrad. I wanted to learn more about the rhinoviruses, which cause the common cold, infectious agents that affect humans with the greatest frequency. At the time, we did not know much about rhinoviruses and I went to join a research group that studied them at the University of Virginia in the United States. We also had to pay attention to the already known coronavirus varieties because they were considered the second most frequent cause of the common cold. These viruses represent 10% of respiratory infections in children, which is significant. At the end of the 1980s, we began to track coronaviruses in people with colds and, since then, these viruses have been on our radar.
Are there recent studies?
In 2019, we published the result of a study carried out with 236 children cared for at the Clinics Hospital at USP in Ribeirão Preto. Out of the more than 200 studied viruses, the coronaviruses, whether alone or in conjunction with the rhinovirus C, were the causes of the most serious infections that more often led to admission to pediatric ICU (Intensive Care Unit). This study only looked at the four coronaviruses that were already endemic in humans, known by the following acronyms: OC43, 229E, HK11 and NL63.
With your long-term knowledge of coronaviruses, did you ever imagine that SARS-CoV-2 could cause a pandemic?
When the news arrived about the new virus in China in December 2019, I thought we would see something similar to what happened in 2002 with SARS-CoV, which caused the Severe Acute Respiratory Syndrome (SARS), or in 2012 with the virus of the Middle East Respiratory Syndrome (MERS), which were respectively restricted to Southeast Asia and the Middle East. It was a surprise that the novel coronavirus spread like wildfire.
– Combat strategy
– Obstacle course
– Breathless emerging economies
– The expected effect of vaccines
Were there no clues about this potential?
The SARS and MERS viruses caused serious illness but had low transmission rates. All of them are very similar in their zoonotic origins. They are the result of spillover, when there is a jump from an animal species to humans. What the coronavirus endemics are teaching us, and which is being confirmed, is that the immune response of antibodies against them is weak. This has been known since the 1960s, when British virologist David Tyrrell (1925–2005), who discovered the coronaviruses, inoculated the virus into the noses of healthy volunteers. The people developed a cold and produced antibodies against the virus. One year later, Tyrrell reinfected the same individuals with the virus and verified that the antibodies barely existed anymore.
Is there a risk that this characteristic could threaten the efficacy of vaccines?
Before responding to this question, I need to take one step back. The immune response can be divided into two parts: one that is controlled by the cells called B lymphocytes and the other by T lymphocytes, which interact and exchange information. The B lymphocytes produce antibodies while the T lymphocytes, after first contact with the virus, recognize the cells it has infected. A subset of T lymphocytes, T CD8, can eliminate the cells that contain the virus. Immunity that is created by the memory T lymphocytes is more long-lasting than that of antibodies. I made this introduction to now provide a response: yes, the way in which the coronaviruses awaken the immune response of antibodies, which is short-lived, can reduce the efficacy of some vaccines.
Which ones could be affected?
The majority of vaccines have been developed to produce antibodies only against the virus’s spike protein, protein S. This is the case with the RNA vaccines, such as that of Pfizer-BioNTech and Moderna, or those that use another virus to introduce into an organism the recipe for making the spike protein of SARS-CoV-2, as with the vaccine made by the University of Oxford/AstraZeneca. These vaccines are more susceptible to losing efficacy because the spike protein, which remains on the surface of the virus, may mutate and, at some point, stop being recognized by the antibodies induced by the vaccines. As viruses multiply, their genetic material is copied and can have errors, which are mutations. Some can cause changes to the proteins and make them unrecognizable by an immune system that was exposed to a prior version of the virus. From an evolutionary perspective, the tendency is for the virus to adapt and become less harmful to the host. This happens because it goes through the Darwinian process of natural selection. They multiply more successfully and are more likely to be surpassed by variants that do not kill the host or those that cause a lighter disease that can result in endemics. We see this all the time in studies with cells and animals. For screening purposes, there is great probability that mutations of the spike protein will occur which, unfortunately, is happening at this time.
From an evolutionary perspective, the tendency is for the virus to adapt and become less harmful to the host
What vaccine would you take?
If I could choose, I would take a whole virus vaccine, such as CoronaVac, made by Sinovac in partnership with the Butantan Institute, or Covaxin, made by Bharat Biotech of India. These vaccines are somewhat “unrefined,” made with the virus perforated and treated with detergent and formaldehyde. They contain all the elements of the virus, as with the flu vaccine. We know the immune response they create. The organism, particularly through the memory T cells, becomes capable of identifying various parts of the virus and not just the spike protein. It is much easier for mutations to occur in a single protein than simultaneously in various proteins. Furthermore, the vaccines made with inactivated whole virus generate a cellular response, by the T lymphocytes, which is longer lasting. Studies carried out in Europe and the United States have shown that between 40% and 50% of people never exposed to SARS-CoV-2 had T lymphocytes capable of destroying it, likely because these cells had previous contact with the endemic coronaviruses and were able to recognize parts that are very similar in the novel coronavirus.
This means that a vaccine of inactivated whole virus could produce a more robust, longer lasting immune response than an RNA vaccine, even though its efficacy is lower?
Exactly. Vaccines were made to prevent illness, not to avoid infection. A classic example is the vaccine against rotavirus. It has practically eliminated cases of severe diarrhea caused by this virus, but it does not prevent infection. Whoever receives a whole virus vaccine against SARS-CoV-2 could still be infected by it, but likely will not get sick nor even know they are infected. There was an unnecessary kerfuffle about the efficacy of CoronaVac, which apparently is very good for avoiding mortality and preventing close to 80% of serious cases and 50% of light symptoms. A vaccine that only stimulates the production of antibodies against the virus’s spike protein can lose efficacy if it moves toward predominating a strain with altered spike proteins.
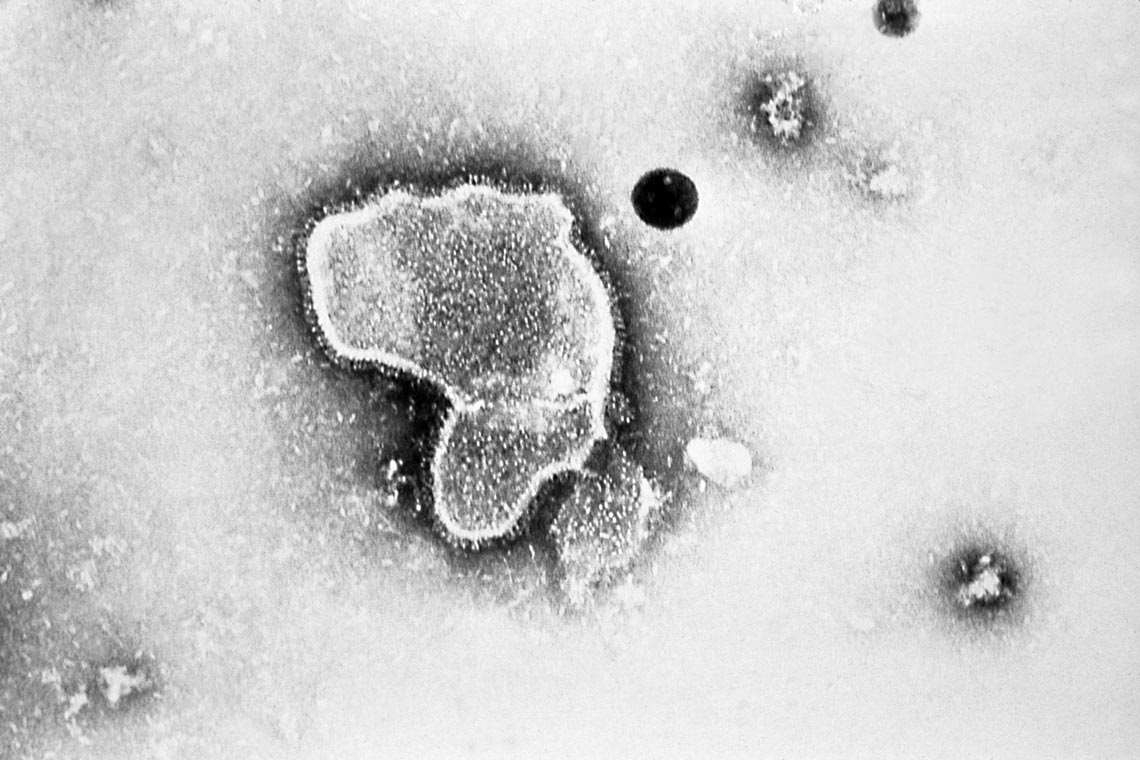
Erskine Palmer / CDC
Copies of the endemic coronavirus OC43…Erskine Palmer / CDCThe emergence of variants shows the importance of monitoring circulating viruses. Has this been done adequately?
No. We need to collect samples and sequence the genetic material of this virus much more than what has already been done, primarily in Brazil. Some countries do a lot of sequencing and know which variants of the virus are out there. In Brazil, we are not yet doing this at adequate levels. There are groups that are carrying out considerable sequencing in São Paulo, Rio de Janeiro, Amazonas, and Rio Grande do Sul, but we should be doing much more and in a more widespread manner. It does not help if we sequence all the viruses in São Paulo and none in Mato Grosso, for example. We should have sentinel clinics throughout the country to collect these samples and sequence them to monitor the spread of variants. We also need to do tests to monitor if these variants are able to escape from the antibodies that are induced by the vaccines.
Does this risk of escape make vaccinating the population more urgent?
Yes, we need to vaccinate a large number of people quickly to prevent these variants from spreading because, the more people who are infected before being immunized, the more the virus will replicate, accumulate mutations, and create new variants.
There are indications of some variants spreading more quickly. Have studies confirmed any of these?
Besides evidence of greater transmissibility in animal subjects obtained with mutation D614G, there is no definitive proof that the emerging variants are more transmissible. For now, there is evidence that some produce greater quantities of the virus in secretions, which would make them more easily transmitted. Therefore, some governments have taken preventative action, prohibiting entry of people coming from places where these variants are circulating. But we cannot yet confirm that all of them are truly more transmissible. Studies have been done using computational molecular modeling that suggest greater transmissibility. But this must be validated in laboratory experiments. Studies of transmissibility are generally done with rats. You put an infected animal in a cage and verify if it infects the healthy animal that is in a neighboring cage and with which it shares the same air. Without these experiments, we cannot know, for example, why a certain variant is spreading significantly, such as in Manaus. It may be very easily transmitted, but it could be that it is more abundant there only because it emerged in that city, where now there are almost no more cases of the variant that caused the first wave.
Only at the beginning of this year, China authorized the entry of the World Health Organization team to investigate the origin of the novel coronavirus. Why is it important to know from which animal it emerged and how it reached humans?
To understand how a spillover happens and so that they can look for ways to avoid others from happening. Spillovers are the result of environmental breakdown caused by human activity. We must reduce the damage by knowing, for example, which species of bats that used to be in the forest, in their natural environment, and now are in the cities. Bats have been on the planet for more than 60 million years and are hosts to many viruses without falling ill because they have an immune system that does not produce much inflammation. If through genetic and computational studies we get to know, in advance, the viruses that these animals carry and the relationship between these viruses and the proteins in human cells, it is possible to remain vigilant, seek to avoid contagion, and prepare early methods for prevention and treatment.
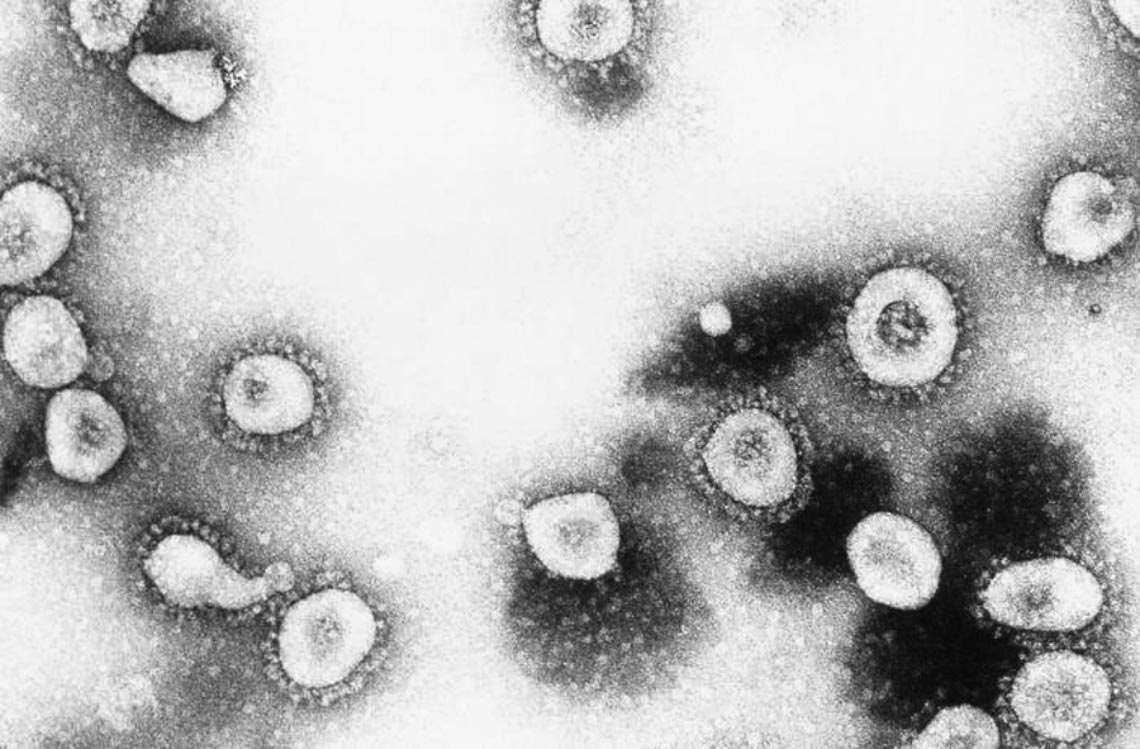
Erskine Palmer / CDC
…and a sample of the respiratory syncytial virus (in humans)Erskine Palmer / CDCWhich centers adequately achieve this zoonotic surveillance?
Very few. One of the top centers is the Duke-NUS Medical School in Singapore. In the United States, there are some centers, such as those at Galveston in Texas and the University of Tulane in New Orleans. In Brazil, unfortunately, there are no institutions that work in this area. We have very good public health institutions, such as the Evandro Chagas Institute in Pará, the Oswaldo Cruz Foundation in Rio de Janeiro and in other states, and the Adolfo Lutz Institute in São Paulo. But they are overwhelmed with day-to-day activities to be able to carry out virus prospecting.
What were the things China and other countries did right and wrong once they discovered the virus was dangerous?
I do not see any grave error on the part of the Chinese. They isolated the virus, sequenced the genetic material, and shared the information. At the beginning, the entire world doubted the virus would spread, wondering, “will it really get here?”. It did. In Brazil, drastic measures should have been taken right from the start, such as tracking at airports, restricting travel, and other actions. But it is difficult. There is political and economic pressure. Something that really created problems was the spread of fake news suggesting that chloroquine or ivermectin could treat the disease. Honestly, I am fearful about the spread of fake news about the vaccines. We have already seen people saying they will not get immunized even if the vaccine is offered to them.
Is there reason for the concern about reinfection?
Every virus that awakens weak immunity in an organism, such as the coronaviruses, can cause reinfection. In the 1960s, David Tyrrell showed how this can happen. What surprises me about the novel coronavirus is that reinfections are happening in a very short time interval, of 60 days, 45 days. I suspect that some of these cases are, in fact, persistent conditions of the virus in the organism.
How does persistence work?
Years ago, we began to study tonsils removed from children when they were enlarged. This causes respiratory problems and even facial deformation. However, the children did not show signs of flu or cold at the time of surgery or in the prior month. In laboratory, we analyzed these tonsils and verified that, in 97% of cases, they were infected with one or more respiratory viruses. We found genetic material and proteins of various viruses in the tissue. When we macerated the tonsils and placed the material in a cell culture, the viruses began to multiply. After this, other groups showed the same result. More recently, we began to study other lymphoid tissues—tonsils, spleen, lymph nodes, thymus, and bone marrow—of people who had died of cardiovascular complications. While these individuals did not die from respiratory issues, we found, in some of the organs, respiratory viruses, such as rhinovirus, respiratory syncytial virus, flu virus, and others.
Vaccines were made to prevent illness, and not to avoid infection. A classic example is the vaccine against rotavirus.
What does this show?
That these people probably had a viral respiratory infection in the past, had colds with coughing and sneezing, and their immune systems beat the infection. But the virus found a cavity where it could live without causing damage to the host. We are investigating the conditions in which infection can be reactivated. I believe that this situation, where the virus lives with the host without causing illness, could be advantageous for both. For the virus, because it can live for longer periods; for the organism, because viral persistence can serve as a stimulus for immunological memory for how to fight the infection.
By simply sequencing the genetic material of the viruses twice and later doing a comparison, it is possible to know for sure if there is reinfection or if it is persistent infection?
Exactly. I am certain of the possibility of reinfection, but I think we need to be more rigorous in its documentation. Something that has not yet been detected with the novel coronavirus, but could happen, is recombination. If the same cell becomes infected with two different strains, the genetic material of each can mix together and create a third.
From the beginning of the pandemic, you led your laboratory to study the novel coronavirus. What did your group discover?
Our most important discovery to this point has been that the novel coronavirus infects defense cells: monocytes, B lymphocytes, CD4 T lymphocytes, and to my surprise, even CD8 T lymphocytes. All these cells are involved in fighting the virus. Some viruses, such as HIV, infect lymphocytes, but it is not known if coronaviruses are also capable of this.
What might be the consequence?
Lymphocytes are cells that fight infections in various tissues. If the virus infects and kills lymphocytes, it can hinder the immune response. But SARS-CoV-2 does not only infect the lymphocytes responsible for fighting it. It invades the lymphocytes intended for other pathogens, which can facilitate other infections. From the beginning of the pandemic, patients with moderate and acute cases of Covid-19 have been observed with lymphopenia, characterized by low lymphocytes in the blood. The cause is unknown. It could be that the reduction occurs due to the lymphocytes having migrated to the infected tissues. We have shown that the virus also kills lymphocytes, which can have another important repercussion. The intense inflammatory response observed in Covid-19 may be the result of a SARS-CoV-2 infection in certain lymphocyte clones. This would make these clones secrete an enormous quantity of cytokines. We do not yet have proof of this.
SARS-CoV-2 infects the lymphocytes responsible for fighting it and also those that target other pathogens
What else have you seen?
In another study, we have supported Fernando Cunha’s group, from the USP Faculty of Medicine in Ribeirão, to demonstrate that SARS-CoV-2 induces another type of defense cell, neutrophils, to release neutrophil extracellular traps, or NETs. When these cells are under stress, such as viral infection, they project DNA fibers into their external environment, which end up capturing pathogens during bacterial and fungal infections. The NETs are very toxic and can cause inflammation. We have seen that the NETs participate in the Covid-19 inflammatory response. The lungs of someone who dies are covered with them in the sites where the virus is present. This finding made way for an approach to attempt to reduce lung inflammation. There are breathing treatments that are based on the use of an enzyme called DNase to dissolve the NETs. In another study, undertaken by Norberto Peporine Lopes, from the Faculty of Pharmaceutical Sciences in Ribeirão Preto, we have seen, through a computational model, that the molecule tenofovir disoproxil fumarate, an antiviral used against HIV, fits very well with the SARS-CoV-2 polymerase. This enzyme acts in the multiplication of the virus’s genetic material. In experiments with cells, we have shown that tenofovir reduced the quantity of virus in hundreds of cases. We reported the result to the Ministry of Health, and we were able to begin a clinical trial, which is currently underway in Ceará, to verify if it will reduce the viral load, the need for hospitalization, and the severity of the illness. With the Dario Zamboni group, we observed that the virus, upon invasion of immune system cells, activates within them the formation of a protein complex called the inflammasome, which triggers the inflammatory response.
How did your interest in viruses begin?
In 1981, I was a 6th year student in medicine at UFC and I was already interested in the biochemistry of viruses. One day, I met a professor of social medicine who told me about a project of researchers at the University of Virginia in the United States. They were doing a survey of infectious diseases, among them viral illnesses, in the slum in Gonçalves Dias in Fortaleza. I sought out the coordinator of the study, Richard Guerrant, and told him that I was studying infectious diseases to become a virologist. He agreed I could participate, and we completed a very thorough study. For two years, we visited people’s homes three times per week to verify if children under 5 years of age were showing symptoms of illness and to collect material for laboratory analysis. I collected good material and identified some viruses. During my doctoral studies at UNIFESP, I received a scholarship to complete the analysis of the material from Fortaleza in the respiratory virus laboratory at the University of Virginia.
What did you observe?
We analyzed a dashboard of respiratory viruses detected in those children. The rhinovirus, which causes colds, was the champion, being five times more common than the others. I had graduated in medicine, but I had not studied this topic, which was not considered an important health issue. But it is. Infection with rhinovirus can trigger asthma attacks, sinusitis, and otitis media. Fifty percent of patients who go to emergency to treat an asthma attack are infected by a rhinovirus. Today, it is believed that this attack is caused by the immune system’s response to this virus. A rhinovirus vaccine can reduce asthma attacks by 50%. At that time, not much was known about this virus, so I decided to study it. It was my first project to show the type of cell that rhinoviruses replicated: the ciliated epithelial cells. Today, I am seeing this virus also reproducing in the lymphocytes of tonsils and other lymphatic organs.
Why is it so difficult to obtain effective antivirals?
The viruses are biochemical parasites with considerable finesse. In my opinion, they are living beings because they replicate, leave behind descendants, and evolve. However, they depend almost completely on another living being, the host cell. As they depend so much on the metabolic pathways of the cell, we need to find compounds capable of inhibiting the replication of the virus without damaging the cell. It has been nearly impossible to separate the pathways that are strictly viral from those that are cellular. There are many antivirals, but they cannot be used. In the experiments, they impede the reproduction of the viruses, but as they compromise the metabolic pathways of the cells, they kill them.


