Almost 40 years ago, immunologist Yoshikazu Kurosawa and his team at a private university close to Nagoya, Japan, revealed to the world that it was possible to manipulate T lymphocytes, the defense system’s soldiers, to counter cancer cells. The first laboratory testing with these modified lymphocytes, which later came to be known as CAR-T lymphocytes or cells, were reported in the journal Biochemical and Biophysical Research Communications on New Year’s Eve 1987. However, it would take more than two decades before their first use on a human being in 2010 with American William Paul Ludwig (1945–2021), who beat leukemia and later died of COVID-19, and many more years for them to be adopted on a wider scale.
– Center in São Paulo will test modified immune cells to treat blood cancer in 81 patients
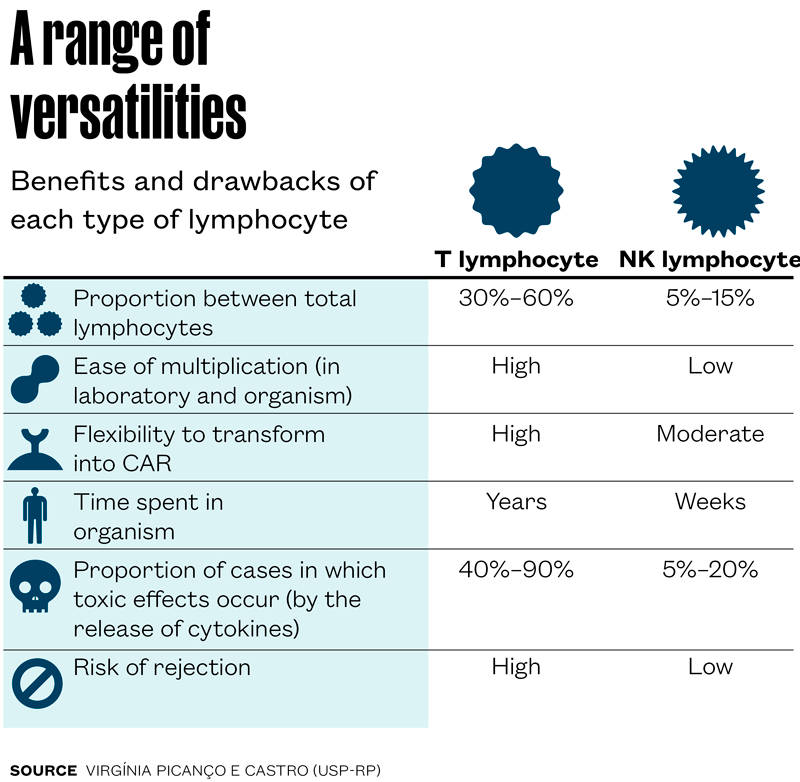
T lymphocytes, though, are not perfect: they can be very toxic and cause a serious form of cytokine release syndrome. Moreover, to avoid rejection they need to be extracted from the patient, and are not always in their best form due to previous cancer treatments. These and other reasons led researchers, including in Brazil, to seek alternatives. The most promising, in the initial human testing phase, is to replace the T lymphocytes with another type of lymphocyte that spontaneously tackles the tumor cells: natural killers (NK).
NK lymphocytes stay in the blood in a lower proportion than T lymphocytes—the former account for between 5% and 15% of the lymphocyte total, while the latter can reach 60%. They recognize tumor cell surface molecules and, like the T, eliminate them with a payload of toxic chemical compounds. One advantage of using NK is that they can be extracted from a healthy person, modified in the laboratory (transformed into CAR-NK), and infused into cancer patients. As these lymphocytes do not recognize the recipient cells as foreign, they can be produced on a larger scale and stored for subsequent administration to different patients as required.
The researchers, however, must manipulate them to stimulate their multiplication prior to infusion, as they proliferate very little in the recipient organism. To overcome this limitation, viral vectors are commonly used to insert genes that codify stimulating molecules, such as interleukin 15 (IL-15), which promote the revival, activation, and expansion of the CAR-NK lymphocytes.
“The manufacture of CAR-NK cells carries a similar cost to that of CAR-T cells. The difference is in the usage. Using a CAR-NK preparation, sufficient material can be obtained to treat between 10 and 100 patients, while CAR-T is used with a single individual,” says hematologist Lucila Kerbauy, of Hospital Israelita Albert Einstein (HIAE) in São Paulo. “We don’t use NK from the patients themselves because due to previous treatments they can become exhausted and dysfunctional,” explains the researcher, who leads one of the Brazilian groups investigating the refinement of these cells to treat different types of cancer.
During her postdoctoral research at the M.D. Anderson Cancer Center in the US, Kerbauy took part in the development of CAR-NK cells used in one of the very few clinical trials now concluded—according to clinicaltrials.gov, only three have been completed. In their trial, hematologist Katy Rezvani and her team used modified CAR-NK lymphocytes to produce IL-15 in 11 patients with lymphoma or chronic lymphoblastic leukemia who had unsuccessfully completed a range of therapies. This was a phase 1 and 2 study to evaluate different doses and toxicities in treatment. According to the results, published in 2020 in The New England Journal of Medicine, no serious toxic effects were identified, and of the 11 people treated, at least six were free of the illness for a number of months. The cancer reappeared in at least one of them, and others underwent new therapies.
Back in Brazil, Kerbauy and her team at HIAE, with the support of FAPESP, worked on the development of two types of CAR-NK cells, personalized for different cancer types: one expresses the CD70 protein against leukemia and lymphoma B lymphocytes; the other expresses the protein BCMA, typical of myeloma B lymphocytes, a blood cancer that afflicts the elderly and leads to the rampant multiplication of plasma cells. “We managed to modify more than 50% of the cells during the production phase. We are now commencing the tests on animals,” concludes the researcher.
At the Ribeirão Preto Blood Center, associated to the University of São Paulo (USP), a group coordinated by biologist Virgínia Picanço e Castro is one step ahead. After helping to develop therapy with CAR-T cells during trials at the Advanced Therapy Unit (NUTERA-RP), in recent years the researcher and her collaborators, also with funding from FAPESP, have been focused on the production of CAR-NK cells to treat leukemia and lymphoma.
The team has produced and tested two types of CAR-NK cells: one was modified to produce an artificial version of the protein interleukin 15 bonded to its receptor, which favors multiplication in culture, and the other with interleukin 27, which increases the elimination power of these cells.
The former were tested successfully against human leukemia cells cultivated in the laboratory and implanted into mice, according to results published in Frontiers in Immunology in 2023. The second type proved effective against human lymphoma in experiments with cells and mice, described in 2024 in the journal Cytotherapy.
The group is currently set to progress the CAR-NK with interleukin 15 and its receptor to the next phase. “We have now mastered the expansion phase and are adapting production for the closed system, without contact with the environment, and following good production practices, as applied at NUTERA,” says biotechnologist Heloísa Brand, doing PhD research under the guidance of Picanço e Castro when she received Pesquisa FAPESP at the laboratory in early June.
“As soon as this phase is concluded, we will repeat the preclinical studies in line with good laboratory practices. These data will make up the dossier for submission to ANVISA as part of the authorization request for a phase-1 test against blood cancers caused by B lymphocytes,” says Picanço e Castro. “Having CAR-NK cells ready for use ‘on the shelf’ may reduce lead times for commencement of treatment,” she adds. As T lymphocytes attack the cells of other organisms, they are used individually and require extraction and preparation four days before the infusion.
The story above was published with the title “Trained killers” in issue 354 of August/2025.
Scientific articles
KUWANA, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochemical and Biophysical Research Communications. Dec. 31, 1987.
MITRA, A. et al. From bench to bedside: The history and progress of CAR-T cell therapy. Frontiers in Immunology. May 15, 2023.
SILVESTRE, R. N. et al. Engineering NK-CAR. 19 cells with the IL-15/IL-15Ra complex improved proliferation and anti-tumor effect in vivo. Frontiers in Immunology. Sept. 24, 2023.
BIGGI, A. F. B. et al. IL-27-engineered CAR.19-NK-92 cells exhibit enhanced therapeutic efficacy. Cytotherapy. Nov. 2024.
LIU, E. et al. Use of CAR-transduced Natural Killer cells in CD19-positive lymphoid tumors. The New England Journal of Medicine. Feb. 5, 2020.
