Eight months after the Sars-CoV-2 virus, which causes COVID-19, was first identified in humans in the city of Wuhan, China, researchers and doctors have yet to figure out the puzzle of how our immune system responds to the pathogen. Knowing exactly how those infected fight and eliminate the virus, and for how long they remain immune to reinfection, are key to the development of vaccines, medications, and even public policy regarding self-isolation measures, quarantines, and lockdowns.
Gradually, the numerous amounts of research about COVID-19 provide the pieces to the puzzle. The full picture, however, remains incomplete. So far, there have been no proven cases of reinfection—to scientists, a sign that those infected do acquire some level of immunity. To what extent and for how long they remain immune is still unknown.
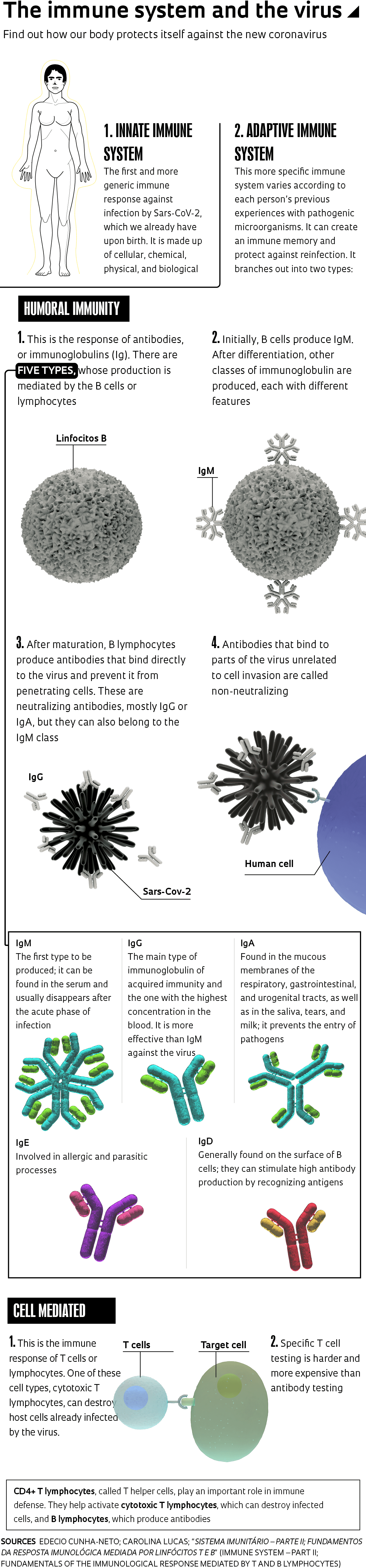
The task of putting this puzzle together is made all the more difficult by the novelty of the virus and the complexity of the human immune system—often compared to an orchestra, to an army, or to a complex piece of machinery. The most popular battalion in this defense force consists of antibodies, including the well-known immunoglobulins G (IgG) and M (IgM), recognized by the quick tests performed in pharmacies and laboratories (see sidebar). Antibodies are proteins dissolved in the plasma and produced by B lymphocytes. Doctors call this sort of defense provided by antibodies “humoral immunity.”
– Times of uncertainty
– Health professionals under emotional stress
– The risk of traveling by plane
– Varying investment in science
– Sharing and expanding knowledge
– Pandemics as allegory
Epidemiological research generally looks for these types of antibodies to check what percentage of a certain population group has been in contact with the virus. “We often say that IgG is almost like an immunological scar, a sign left behind by a virus,” explains immunologist Jorge Kalil, a professor of Clinical Immunology and Allergy at the University of São Paulo School of Medicine (FM-USP) and director of the Immunology Laboratory of the Heart Institute (INCOR).
A Chinese study published on June 18 in the journal Nature Medicine, however, noted that 37 people who were known to have been infected with Sars-CoV-2, but remained asymptomatic, had lower blood levels of IgG and neutralizing antibodies (which prevent the virus from penetrating cells) than those who had moderate symptoms, despite having detectable levels of the virus in their blood for longer. In addition, two or three months after acute infection, the rates of these antibodies became undetectable in 40% of asymptomatic cases. According to the research, 12.9% of symptomatic patients became seronegative.
The study results raised questions as to whether those with the mild form of COVID-19 might be more susceptible to reinfection. Experts claim we do not yet have an answer. One possibility is that tests failed to detect levels of antibodies that, while low, might still be enough to fight the virus. Either way, the authors of the paper, from Chongquing Medical University, believe the results help show the risks of COVID-19 immunity passports, which have been considered by certain governments as a way to allow travel for those who have already recovered from the infection.
“Basing immunity passports on the presence of IgG antibodies has already been discarded,” assesses infectious disease expert Nancy Bellei, from the Federal University of São Paulo (UNIFESP). “We’ve found that, for many patients who had the disease, their IgG antibodies no longer recognizes viral antigens.” Bellei, an expert on respiratory virus research for decades, explains that the results of this Chinese study are not exactly surprising, as other research seems to point in the same direction—such as a study released in mid-July by researchers from King’s College London in England.
“Respiratory viruses do not usually cause permanent immunity and people infected with them do not usually retain stable positive immunoglobulin levels long term,” says the researcher. “Despite being new to the human population and having pathogenic effects on other tissues beyond the respiratory system, Sars-CoV-2 is still behaving and being classified as a respiratory virus,” she points out.
But do low or non-existent levels of IgG antibodies in the plasma of those previously infected—even if asymptomatic—necessarily indicate a total lack of immunity to the new coronavirus? The answer to that question, according to infectious disease experts and immunologists, is likely no. Two recent articles, published in the preprint repositories medRxiv and bioRxiv, show that some people can develop a strong antigen-specific T lymphocyte response to Sars-CoV-2, despite being seronegative. Lymphocytes are one type of defense cell in the human body.
The first article, authored by scientists from the University of Strasbourg, France, studies seven families with at least one member recovering from the moderate form of COVID-19. Six out of eight family members who later developed symptoms of the disease showed T cell responses, but no antibodies against the new coronavirus.
The second study, by researchers from the Karolinska Institute, in Sweden, investigated the memory T cell response in different groups of people: healthy blood donors, who donated before or during the pandemic; people who shared a home with recovering individuals and were exposed to the disease during the symptomatic phase; and people who were recovering from the disease, who had had mild to no symptoms of the severe form of COVID-19. The scientists also observed a robust T lymphocyte response even without antibodies.

Léo Ramos Chaves
Scientists from Bio-Manguinhos, Rio de Janeiro, work on the development of a vaccine against the novel coronavirusLéo Ramos Chaves“The results of these studies, added to the lack of cases of reinfection to date, give us some peace of mind that we will likely have a certain degree of protection, at least for some time. As for is the duration of immunity, only time will tell,” considers infectious disease expert Reinaldo Salomão, from the UNIFESP School of Medicine, principal investigator of a FAPESP-supported study on the inflammatory and immunological responses of patients with severe and moderate COVID-19.
Salomão points out that the tests themselves may be to blame for the non-detection of IgG in individuals who became infected, as shown by the studies carried out so far—they may not be sensitive and specific enough for Sars-CoV-2. “This also happened with HIV. We are currently in our fourth generation of HIV tests and they have improved significantly.”
On the other hand, experts point out that even positive IgG test results do not necessarily mean the individual is protected against the virus, since the tests cannot determine whether or not neutralizing antibodies, which prevent the new coronavirus from penetrating human cells, were produced. They explain that the presence of IgG in the blood is not necessarily related to the existence of neutralizing antibodies.
The first study to point to the possibility of a vigorous human cell response against the new coronavirus, according to immunologist Edecio Cunha-Neto, from INCOR and FM-USP, was published last May in the journal Cell, by scientists from the La Jolla Institute for Immunology in San Diego, California, United States.
In addition to observing a strong cellular response to proteins that simulated Sars-CoV-2 in a group of 20 adults who recovered from COVID-19, they also saw a positive reaction in 40% of the blood samples taken in 2019, before the spread of COVID-19, used in the study as the control. This means there was a cellular immune response to peptides—protein fragments—that resembled the novel coronavirus peptides in the blood of people who had never been in contact with the virus.

Léo Ramos Chaves
A health professional collects blood for a quick COVID-19 serology testLéo Ramos Chaves“The only explanation is that these memory T cells in these individuals appeared after they were in contact with a very similar pathogen,” says Cunha-Neto. “This pathogen does exist: it is the endemic or seasonal coronavirus, which causes a mild cold.” Cunha-Neto explains that about 50% of the protein sequence of endemic coronaviruses are identical to Sars-CoV-2. “The paper from the University of Strasbourg had already described an intense and frequent reactivity to endemic coronaviruses in all family members that caught COVID-19 and in 80% of the healthy people tested,” says Cunha-Neto.
In a July 7 comment posted in Nature Reviews Immunology, scientists Alessandro Sette and Shane Crotty, coauthors of the Cell paper, claimed that over 90% of the human population is seropositive for at least three of the four types of endemic coronaviruses that circulate around the world. They also said that the cross-reactive response of memory T cells could interfere with vaccination results, leading to a better or faster immune response, for example. However, researchers warn that it could also be a confusing factor, especially for phase 1 trials in vaccine development. This is because phase 1 trials cover smaller groups of people, and their previous contact, or lack thereof, with endemic coronaviruses could lead to different conclusions.
In an effort to prevent a cross-reactive response and test if there is specific and effective cellular immunity to the new coronavirus, Cunha-Neto and his research group are preparing an experiment in which they will select the peptides used to verify cellular response, separating the parts that are exclusive to Sars-CoV-2 from those identical to other coronaviruses. “Only then will we be able to tell whether there really are seronegative COVID-19 patients with specific cellular response to Sars-CoV-2 and how often they occur,” explains the researcher. If proven, this could indicate that measuring numbers of infected or immune patients based on antibody tests may underestimate the actual number of those infected, argues Cunha-Neto.
“While the body only produces large amounts of antibodies before the virus is eliminated, later reducing their levels in the bloodstream until they sometimes become undetectable, memory T cells remain for decades,” he claims. According to Cunha-Neto, patients who had Severe Acute Respiratory Syndrome (SARS) nearly 20 years ago, caused by Sars-CoV-1—a “cousin” of Sars-CoV-2—have few antibodies, but considerable amounts of memory T lymphocytes relating to the original virus, which become vigorously active upon further contact with the antigen. “We currently believe that long-term memory and defense against Sars-CoV-2 are highly dependent upon T lymphocytes. Thus, an effective vaccine should induce the production of both neutralizing and non-neutralizing antibodies, and CD4+ and CD8+ T lymphocytes.”
At INCOR, Kalil is also developing research on 220 patients who had the disease and were able to eliminate the virus. He is studying the response of antibodies and T lymphocytes to define specific targets for the development of a Brazilian vaccine. “We will probably be working on our immunizing agent using virus-like particles, or VLP. We are working to figure out what could induce an improved immune memory,” says the expert. “We do not intend to use the entire protein from the viral spike [the projections that line the pathogen], but isolate fragments to elicit a balanced response from T and B defense cells, which produce antibodies.” With luck and hard work, it can become one more piece that could help assemble the puzzle of immunity against the new coronavirus.
Understand how the body reacts when attacked by an unknown pathogen
The first response of the immune system against infection by Sars-CoV-2, as well as by new viruses and bacteria, comes from so-called innate immunity, which is more generic and present in every human being from birth. “When a patient has a very strong innate response and eliminates the virus, they won’t even experience symptoms,” says infectious disease expert Edecio Cunha-Neto, from the Heart Institute (INCOR).
When innate immunity is unable to annihilate the pathogen within a few days, the body activates its so-called adaptive immunity, which is more specific and varies according to a person’s experiences with pathogenic microorganisms, creating an immune memory. Adaptive immunity involves both antibodies, produced by B cells or lymphocytes—so-called humoral immunity—and T lymphocytes, cellular immunity. The first cells to be activated when the innate immunity fails to resolve the issue are CD4+ T cells. “When this occurs, they act directly on the pathogen and activate CD8+ cytotoxic T cells or B lymphocytes,” explains Cunha-Neto.
Cytotoxic CD8+ T lymphocytes activated by CD4+ T cells become capable of destroying cells that have already become infected by the pathogen, while neutralizing antibodies prevent the virus from penetrating healthy cells. “In most cases, before you have a specific antibody for the virus, you have a specific T cell,” explains the INCOR researcher. Sometimes, the virus is eliminated before any antibodies are produced, since their production takes about 7 to 14 days.
Immunoglobulin M (IgM) antibodies are produced in the acute phase of the infection, while immunoglobulin G (IgG) antibodies show up later, when the individual would theoretically already be protected. Each different pathogen that attacks the body triggers the production of specific IgG and IgM antibodies, among others. There are three other immunoglobulin classes: IgA, IgE, and IgD (see chart).
Depending on the type of virus, IgG levels can remain detectable for months or years. But that does not seem to be the case for Sars-CoV-2: some research indicates that they can become undetectable in two or three weeks.
In a non-peer reviewed paper published in June on the medRxiv platform, researchers from Yale University, United States, describe the results of a longitudinal study of 103 patients who tested positive for COVID-19, compared to 108 control subjects who had tested negative for the disease. They observed the immune response to the virus and each person’s clinical trajectory.
Their two main conclusions were that more severe cases were associated not only with a higher and more prolonged viral load, but with a dysfunction of the immune response, which at first is slower to control Sars-CoV-2 and then ends up triggering an exaggerated inflammatory response. Furthermore, there are immunological markers in the blood already detectable in the initial phase of the disease that can predict the patient’s clinical trajectory.
“This may help guide treatment and clinical interventions,” says Brazilian infectious disease expert Carolina Lucas, the first author of the paper, currently working on her postdoctorate at Yale. “This study shows that we must not only focus on controlling the virus, but also excessive inflammatory responses.”
Projects
1. Prospective cohort study to assess clinical, virological and host response aspects in patients with Covid-19 (no. 20/05110-2); Grant Mechanism Regular Research Grant; Principal Investigator Reinaldo Salomão (UNIFESP); Investment R$143,100.20.
2. INCT 2014: Immunology investigation (no. 14/50890-5); Grant Mechanism Thematic Project; Principal Investigator Jorge Elias Kalil Filho (USP); Investment R$3,980,221.36.
3. Developing a vaccine against SARS-CoV-2 using VLPs (no. 20/05146-7); Grant Mechanism Regular Research Grant; Principal Investigator Gustavo Cabral de Miranda (USP); Investment R$178,100.00.
4. Mapping epitopes of the SARS-CoV-2 virus for T lymphocytes and the B-lymphocyte receptors for the spike protein (no. 20/05256-7); Grant Mechanism Regular Research Grant; Principal Investigator Jorge Elias Kalil Filho (USP); Investment R$154,865.00.
Scientific articles
LONG, Q. et al. Clinical and immunological assessment of asymptomatic Sars-CoV-2 infections. Nature Medicine. June 18, 2020.
SEOW, J. et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv (preprint). July 11, 2020.
GALLAIS, F. et al. Intrafamilial exposure to SARS-CoV-2 induces cellular immune response without seroconversion. medRxiv (preprint). June 22, 2020.
SEKINE, T. et al. Robust T-cell immunity in convalescent individuals with asymptomatic or mild Covid-19. bioRxiv (preprint). June 29, 2020.
GRIFFONE, A. et al. Targets of T-cell responses to SARS-CoV-2 coronavirus in humans with Covid-19 disease and unexposed individuals. Cell. May 14, 2020.
LUCAS, C. et al. Longitudinal immunological analyses reveal inflammatory misfiring in severe Covid-19 patients. medRxiv (preprint). June 27, 2020.