The revolutionary gene-editing technique known as CRISPR-Cas9 is bringing the prospect of treating diseases by altering and replacing genes ever closer to reality. The result of its first use in humans was described in an article in the New England Journal of Medicine in September 2019. Immunologist Deng Hongkui and his team at Peking University in China performed the pioneering test on a 27-year-old man with HIV and leukemia, a type of cancer caused by the proliferation of immature defense cells. After controlling the illnesses with medication, the researchers submitted the patient to an innovative treatment in 2017. Both leukemia and HIV affect the lymphocytes, defense cells that attack invading organisms and diseases. Since the solution to both problems was to restore the production of healthy lymphocytes, the doctors decided to combat the two illnesses at the same time with a special transplant. They took cells from a donor’s bone marrow, but before transferring them to the patient, they used CRISPR to deactivate a gene containing the recipe for a protein HIV uses to invade lymphocytes. The aim was to restore the production of healthy defense cells that are immune to the virus, as happened in 2008 with Timothy Ray Brown, known as the Berlin Patient, after he received bone marrow from a donor who naturally did not produce the invasive protein.
The procedure was a partial success. The researchers transplanted a mixture of edited and unedited cells because it was not possible to alter them all. A year and a half later, the patient’s leukemia remained in remission and the new marrow continued to produce healthy lymphocytes, even though only 5% of them had been genetically edited. “The test was designed to assess the safety and viability of the transplant,” Deng told Pesquisa FAPESP by email. The experiment worked as a proof of principle and suggested that it is possible to perform the procedure without harm. Before assessing its effectiveness as a strategy for conquering HIV, however, scientists need to increase the efficiency of the editing procedure and improve the transplant protocol—ideally, all lymphocytes would become immune to the virus. “We decided to improve the technique before treating other patients,” said the immunologist.
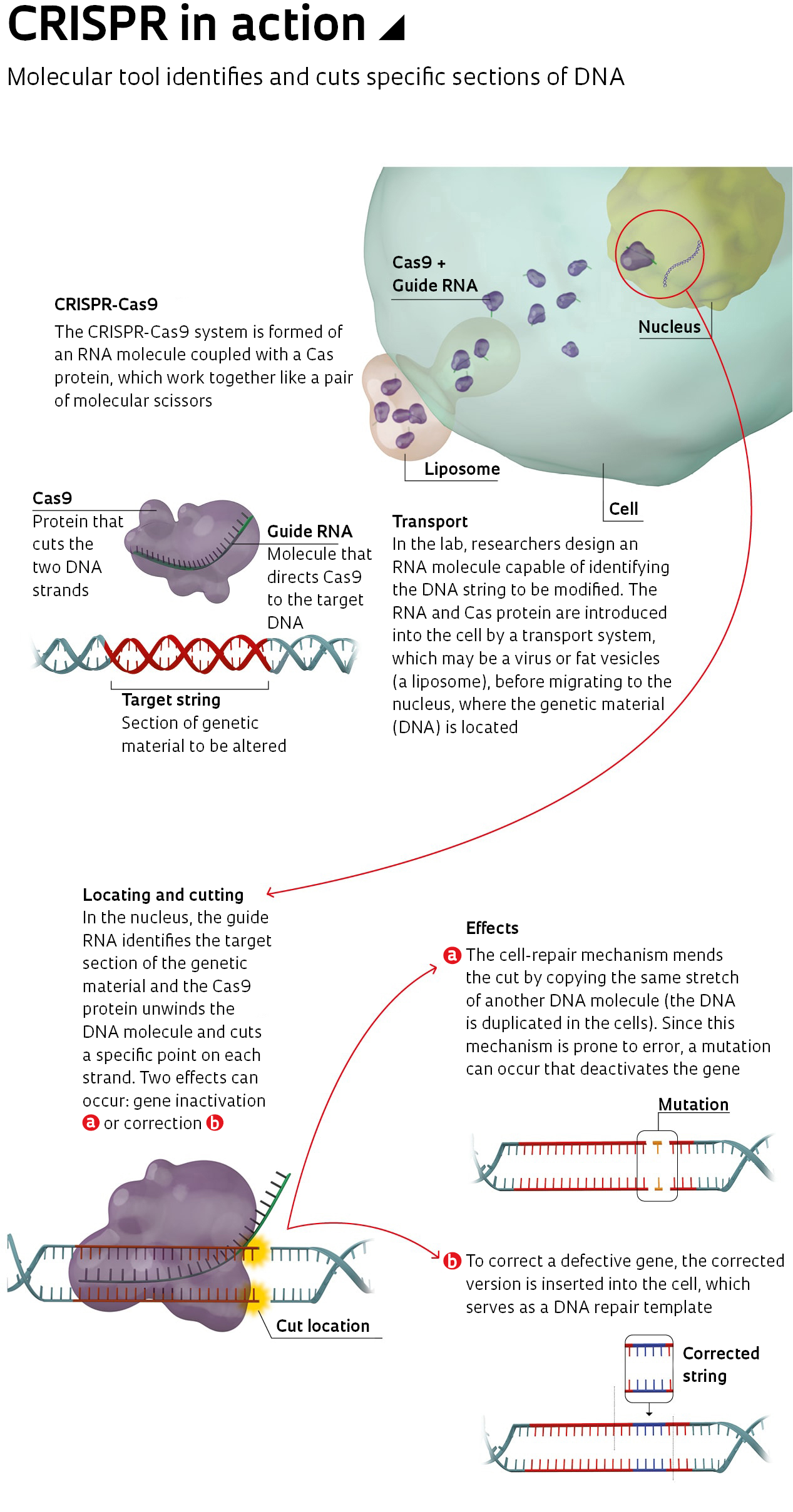
CRISPR is neither the first nor the only way to modify or inactivate genes that has been tested on humans. The idea that it may be possible to cut cellular DNA at specific points and alter it—inserting a new gene or deactivating one that has an undesired effect—came from the discovery of a particular defense system used by bacteria. In the 1960s, American researchers Hamilton Smith and Daniel Nathans (1928–1999) and Swiss scientist Werner Arber identified proteins in these organisms called nucleases, which act as molecular scissors, cutting the genetic material (DNA) of invading viruses at specific points. The discovery earned the team the 1978 Nobel Prize for Medicine and led to the development of various cellular DNA editing strategies.
Two techniques proposed in the 1990s and 2000s made use of the findings: editing with zinc finger nucleases (ZFN) and with transcription activator-like effector nucleases (TALEN). Both use an artificial protein formed by joining two others: one that identifies the stretch of genetic material, and another that cuts it. They are accurate and are currently being tested on animals and humans, with a dozen clinical trials involving ZFN and half a dozen with TALEN (see report). However, there is one obstacle. Proteins are large, complex molecules, which are difficult to produce in a lab. Another complication is that a new protein has to be designed for each target DNA section.
This is where CRISPR prevails. It also uses a hybrid molecule, but formed of a protein (the Cas) and RNA, which is much smaller and easier to design in a lab (see infographic above). In 2012, American biochemist Jennifer Doudna, from the University of California, Berkeley, USA, and French geneticist Emmanuelle Charpentier, now working at the Max Planck Institute in Germany, created a simplified version of the CRISPR-Cas system and showed that it worked in DNA tests. The following year, Chinese-American biochemist Feng Zhang of the Broad Institute, USA, used the technique to manipulate the DNA of human cells. Published in the journal Science, the results sparked a global race to master the technique—and a dispute between Berkeley and Broad over intellectual property rights (see Pesquisa FAPESP issue no. 269).
“Producing short RNA sequences in the lab is cheap and easy, which makes CRISPR more versatile and accessible than any other gene-editing technique,” says geneticist Carlos Menck of the University of São Paulo (USP). In partnership with researcher Clarissa Rocha of the Federal University of São Paulo (UNIFESP), he uses CRISPR to identify genes that make tumor cells resistant to drugs.
Because it is so easy and versatile, researchers around the world quickly began testing CRISPR on plants and animals with a wide range of objectives, from improving food production to creating models for studying human diseases. Mice, rats, rabbits, pigs, dogs, and monkeys have already been genetically altered with CRISPR, many through pioneering work by researchers in China. Less than five years after the articles were published in Science, studies appeared showing that it is possible to correct defective genes in human embryos, and human experiments began.
Human testing
In addition to the Chinese case, which was the first to present data on human tests in a scientific publication, another five were reported more recently. In early November, a team led by physician Edward Stadtmauer, from the University of Pennsylvania, USA, published early data on the use of defense cells that had been given an extra gene using CRISPR that told them to attack two types of tumors (multiple myeloma and sarcoma), while others that stop the action of these cells were deactivated. Six months after treatment, the patients had suffered no serious side-effects. One woman with myeloma had improved, and a second patient’s sarcoma stopped progressing. There are not yet any results for a third case, the researchers reported at the American Society of Hematology’s annual meeting in 2019.
American pharmaceutical company Vertex Pharmaceuticals and Swiss gene-editing company CRISPR Therapeutics also announced in November that they have had early success using CRISPR to treat a patient with beta thalassemia and another with sickle cell anemia, both genetic diseases that alter the patient’s hemoglobin, the protein that carries oxygen in the blood. The cases are part of clinical trials in which 45 participants are receiving cells of their own marrow that have been edited to treat the disease. Months after the treatment finished, the patient with beta thalassemia no longer needed blood transfusions and the woman with sickle cell anemia had no organ lesions caused by blood vessel blockages, usually caused by an accumulation of deformed red blood cells. “These preliminary data support our belief in the potential of these therapies to have a meaningful benefit for patients following a one-time intervention,” said Samarth Kulkarni, president of CRISPR Therapeutics, in a press release.
For the treatment, the researchers used CRISPR to introduce a defect that deactivated the BLC11A gene. They were thus able to reactivate the production of fetal hemoglobin, which is synthesized during intrauterine life. Even in small amounts, fetal hemoglobin reduces the harmful effect of defective hemoglobin. In Brazil, a team led by hematologist Fernando Costa, from the University of Campinas (UNICAMP), is trying to reproduce the treatment, with one difference. Instead of inducing unknown mutations in BLC11A like the American and Swiss companies did, he and biologist Priscila Martin chose to promote a specific alteration identified in the Brazilian population in the 1980s. This mutation increases the production of fetal hemoglobin during adulthood, without affecting other genes. Costa and Martin have already introduced the alteration into human cell lines, which started to produce fetal hemoglobin in greater quantity. The group is now attempting to repeat the procedure in mice with sickle cell anemia. “If the amount of fetal hemoglobin reaches levels close to 25% of the total hemoglobin, it is possible that lesions caused by vessel obstruction will no longer occur,” reports Costa.
As of the beginning of this year, 16 clinical trials were in progress—including the two by Vertex and CRISPR Therapeutics. These are initial tests, designed to evaluate the safety, and to a certain extent the efficiency, of gene editing with CRISPR. Most of them (11) use the technique to change how defense cells work, causing them to attack different types of cancer (lymphoma, leukemia, esophagus, stomach, and lung). The rest aim to mitigate or correct damage caused by hereditary diseases linked to a genetic defect, such as beta thalassemia and sickle cell anemia.
Ten tests are ongoing at hospitals and research institutes in China, and five at American institutions. This number reflects China’s advance in biotechnology and medicine, which has already led some experts to speculate, perhaps prematurely, that the scientific dispute between the Americans and Chinese could become similar to the Cold War between the USA and the Soviet Union. Since 2013, published articles related to CRISPR have increased 100-fold. Pubmed, the world’s largest database of medical articles and books, received 29 new articles on CRISPR-Cas9 in 2013, and 3,221 in 2019. Of the 9,700 papers published between 2013 and 2019, 27% have at least one author from China and 29% have at least one from the USA.
Despite the growing amount of research in the area, it is still too early to know if the technique will work on humans. For now, there has only been preliminary data reported from six cases. The only completed test on humans involved 16 participants with esophageal cancer, conducted at the Hangzhou Cancer Hospital in China. The results, however, were not published. Despite this, many believe that CRISPR will make gene therapy a reality within the next few years.
“CRISPR is already being used to treat diseases in humans. This is not a hypothetical scenario,” says molecular biologist Graham Dellaire, of Dalhousie University in Canada, who studies CRISPR and mechanisms to fight tumor cells. In recent years, he and several colleagues have commented on ethical issues related to gene editing, an even more pressing topic since Chinese biophysicist He Jiankui claimed to have created the first babies edited by CRISPR in November 2018 (see). In December 2019, He was sentenced to three years in prison for illegal medical practices.
First steps
In Brazil, researchers are already testing CRISPR on a range of applications, from cancer treatment to fighting parasites such as Trypanosoma cruzi, which causes Chagas disease. The most advanced results to date were achieved by physiologist Guilherme Baldo, from the Federal University of Rio Grande do Sul (UFRGS). At the Gene Therapy Center of Porto Alegre General Hospital, Baldo and his group used the technique to develop an experimental treatment for mucopolysaccharidosis, a rare genetic disorder that affects one in 100,000 people, damaging the organs and impairing brain development.
A defect in the alpha-L-iduronidase gene causes sugars called mucopolysaccharides to accumulate, causing cell damage. The most effective treatment is a bone marrow transplant, which requires destruction of the defense system and must be performed before the age of two to reduce the risk of intellectual disabilities. Another option is to replace the enzyme, but this approach does not prevent neurological damage and can cost up to R$1 million per year.
Using CRISPR, the researchers from Rio Grande do Sul have already corrected the alteration in human cell cultures and in mice. The therapy restored the gene’s function in 4% of lung and heart cells. A month later, the animals were producing 7% to 8% of the amount of enzyme synthesized by a healthy organism, according to an article published in the Journal of Controlled Release in 2018. “Although the efficiency is still low, it is likely that it is already enough to prevent the disease from progressing. Children who synthesize up to 2% of the enzyme do not suffer intellectual disabilities,” says Baldo. He is looking for ways to increase the availability of the enzyme in the brain and is planning new experiments on animals before the treatment is safe to test on people.
Xenotransplantation
CRISPR could also help with the lack of organs available for transplant. One potential source is pigs, whose organs are similar in size to humans. To make transplantation between species (xenotransplantation) viable, scientists need to eliminate the risk of organ rejection and disease transmission. In 2015, George Church, a geneticist from Harvard University, neutralized 62 porcine endogenous retroviruses that could be harmful to humans, a task that would have been unimaginable before CRISPR.
At USP’s Human Genome and Stem Cell Research Center (HUG-CELL), a team led by surgeon Silvano Raia and geneticist Mayana Zatz is attempting to deactivate the three genes primarily responsible for triggering the host’s defense system, which causes the body to reject the transplanted organ. Biologist Luiz Caires and other members of the group have already shut off one of these genes in pig fetal cells. “The technique is still not that efficient, but we are improving it,” he says. The aim is to deactivate all three genes by September.
The next step will be to extract the DNA from these cells and transfer it into an egg whose nucleus has been removed. “The eggs will be implanted in females to create genetically modified offspring,” says Zatz. By creating genetically modified pig kidneys for humans, the number of people requiring hemodialysis while waiting to receive a kidney from another person (homologous transplant) could be greatly reduced. “In Brazil, 126,000 people are waiting for a kidney transplant and undergo hemodialysis on a regular basis, costing the public health system R$2.8 billion per year,” says Raia, the first physician in the world to perform a liver transplant from a living donor.
In another laboratory at HUG-CELL-USP, geneticist Maria Rita Passos Bueno and her team are using CRISPR to investigate the causes of cleft lip and cleft palate. Alterations in almost a dozen genes can cause this birth defect, which affects facial formation. There are cases, however, of babies born with a cleft lip despite all of their genes being healthy. As part of a research fellowship supervised by Passos Bueno, biologist Lucas Alvizi used a version of CRISPR to identify a possible new cause of the problem. In tests on zebrafish, the group found that the congenital defect could result from hyperactivation of the MIR152 gene. In an article posted on the BiorXiv repository, they describe how this could be caused by low oxygenation during pregnancy.
At USP’s Ribeirão Preto campus in the interior of São Paulo State, teams led by molecular biologist Geraldo Aleixo Passos and immunologist Eduardo Donadi use CRISPR with a different strategy. Instead of correcting gene alterations, they trigger them. The objective is to understand how defects that deactivate the autoimmune regulator gene (AIRE) lead to the development of autoimmune diseases, such as type 1 diabetes.
In mammals, this gene is most active in the thymus, a gland located in the thorax that eliminates defense cells (immature T lymphocytes) capable of attacking the body itself. As reported in Frontiers in Immunology in 2018, the researchers observed that when generating mutations that switch off the AIRE gene, the thymus cells changed the activation profile of the genes and stopped physically interacting with immature lymphocytes, inhibiting the elimination of those that attack the organism. “The CRISPR technique,” says Passos, “is helping us to better understand how the AIRE gene prevents autoimmune diseases.”
Development of an exciting new tool In less than 30 years, a bacterial defense system was adapted to manipulate human genes1987
In the genome of the bacteria Escherichia coli, Yoshizumi Ishino and other researchers at Osaka University, Japan, identify repeated DNA sequences that later come to be known as CRISPRs, short for clustered regularly interspaced short palindromic repeats
1993
Francisco Mojica and his colleagues at the University of Alicante, Spain, find CRISPR sequences in the genome of the archaea Haloferax mediterranei. CRISPR sequences are later found in the genomes of other bacteria and archaea, suggesting that they play an evolutionary role
2002
At Utrecht University in the Netherlands, Ruud Jansen and his team identify genes adjacent to the CRISPR sequences, known as Cas (CRISPR-associated genes). These genes encode proteins that interact with the CRISPRs
2005
At almost exactly the same time, teams led by Mojica in Spain, Christine Pourcel at the University of Paris, and Alexander Bolotin from the Institut National de la Recherche Agronomique (French National Agricultural Research Institute) realize that the stretches of DNA between CRISPR sequences are similar to those of viruses that attack bacteria. The conclusion is that CRISPRs may be a defense system against viruses. In the bacteria Streptococcus thermophilus, Bolotin identifies the Cas9 gene, which encodes a protein that cuts the two strands of DNA at specific points
2007
Philippe Horvath and other researchers from Danish food company Danisco demonstrate that CRISPRs are part of a bacterial defense system that integrates the DNA of the virus into the bacterium, functioning as a sort of memory of the invader
2008
John van der Oost and his team at Wageningen University in the Netherlands show that the strings separating the CRISPR sequences generate a small molecule of RNA (single-stranded genetic material) that guides the Cas protein to the foreign genetic material. At Northwestern University, USA, Luciano Marraffini and Erik Sontheimer discover that the RNA’s target is the invader’s DNA
2010
Microbiologist Sylvain Moineau and his team at Laval University in Canada find that the CRISPR-Cas9 system breaks the two strands of DNA at a specific point
2011
At Umeå University in Sweden, French geneticist Emmanuelle Charpentier confirms that the RNA that guides the Cas9 protein to its target is double-stranded, made up of two RNA molecules. A group led by Virginijus Siksnys at Vilnius University, Lithuania, copies the DNA of Streptoccocus thermophilus that encodes the CRISPR-Cas system and inserts it into the genome of the bacterium Escherichia coli. The system remains active and destroys the DNA of invading viruses
2012
Siksnys’s group describes how Cas9 works and shows that the guide RNA can be manipulated to locate predetermined targets. In partnership with Jennifer Doudna of the University of California, Berkeley, USA, Charpentier, now at the Max Planck Institute in Germany, achieves similar results to Siksnys. Doudna and Charpentier’s groups also show that it is possible to create a single-stranded synthetic RNA to guide Cas9, simplifying the method. In May, the University of California group file for a US patent for using CRISPR-Cas9 to edit genomes. In December, Feng Zhang of the Broad Institute and George Church of Harvard University also apply for a patent for the technique, initiating a still-unresolved dispute
2013
Feng Zhang adapts CRISPR-Cas9 to edit mammalian genomes and tests it on human cells
2014
At Nanjing Medical University, China, Jiahao Sha’s team breeds monkeys with genes edited by CRISPR
2015
In a step towards transplanting a pig organ into a human, Church and his team at Harvard disable 62 retroviruses in the pig genome
2016
At Sichuan University, China, You Lu and his team test the CRISPR-Cas system in humans, switching off a gene to stimulate defense cells to fight lung cancer
2017
Ha Youn Shin and colleagues at the USA’s National Institutes of Health identify off-target alterations caused by CRISPR. At Oregon Health and Science University in the USA, Shoukhrat Mitalipov and his team use the technique on human embryos to correct a mutation that causes heart disease
2018
He Jiankui, from the Southern University of Science and Technology in China, announces that the first human babies have been born with genomes edited using CRISPR. He is then banned from the university, and in December 2019, is sentenced to three years in prison
Sources Broad Institute; Yshino, I., et al. Journal Of Bacteriology. 2018; Nature Biotechnology. 2019.
Projects
1. National production of genetically modified pigs for human organ xenotransplantation (nº 18/14275-5); Grant Mechanism Research Partnership for Technological Innovation Program (PITE); Principal Investigator Silvano Mario Attilio Raia (USP); Investment R$3,748,623.36.
2. HUG-CELL – Human Genome and Stem Cell Research Center (nº 13/08028-1); Grant Mechanism Research, Innovation, and Dissemination Centers (RIDC); Principal Investigator Mayana Zatz (USP); Investment R$43,461,955.95.
Republish