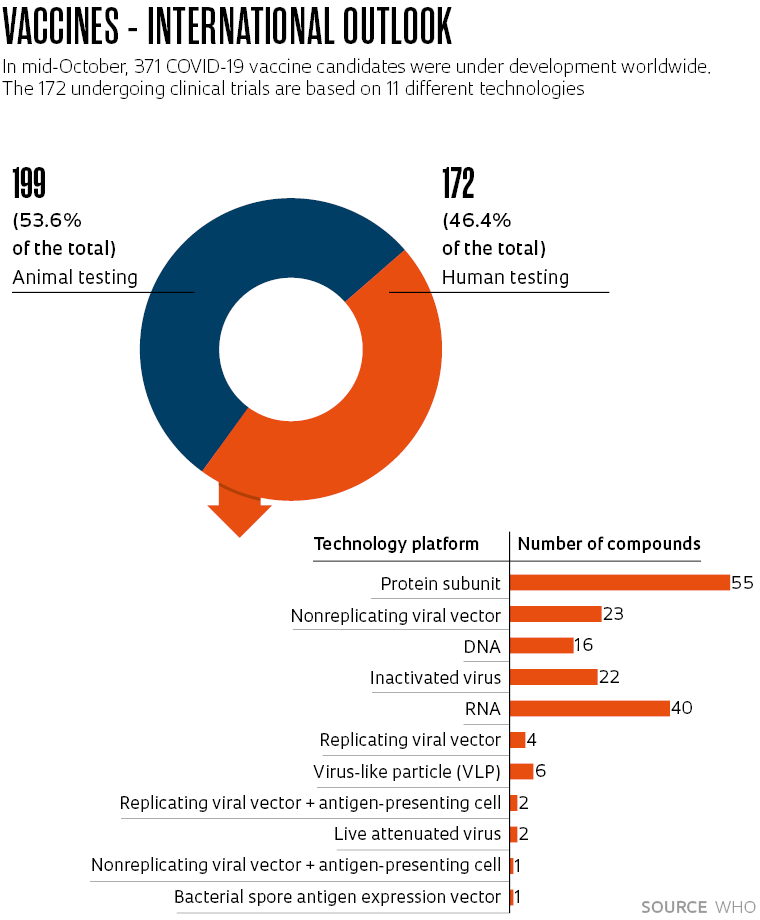On October 3, the Brazilian Health Regulatory Agency (ANVISA), responsible for regulating food and medicine in the country, authorized the first COVID-19 vaccine candidate developed entirely in Brazil to begin human trials. The compound is known by the acronym SpiN-Tec-MCTI-UFMG. Developed by researchers at the Vaccine Technology Center (CTVacinas) of the Federal University of Minas Gerais (UFMG), the plan is to start administering it to volunteers later this year. The trial will be conducted in Belo Horizonte in two stages, involving a total of 432 participants.
In phase 1 of the clinical trial, 72 adult volunteers who have already been immunized against the novel coronavirus will be separated into three groups to receive UFMG’s vaccine as a booster dose. Participants will be given a low, intermediate, or high dose, depending on which group they are in. The objective is to verify whether the compound is safe and to define which dosage triggers the greatest immune response. If everything goes as planned, phase 2 will start in February next year, during which another 180 people already vaccinated against SARS-CoV-2 will receive a dose of SpiN-Tec. Their antibody levels and the response of their immune cells (T lymphocytes) will be compared to an equal number of participants given a booster of a different vaccine already in use in Brazil. At this stage, the aim will be to investigate whether UFMG’s compound induces a greater immune response than other widely adopted vaccines, thus paving the way for the final stage of human testing: phase 3 clinical trials.
“Brazil has never made a human vaccine from scratch,” says immunologist Ricardo Gazzinelli, head of CTVacinas and leader of the team that developed SpiN-Tec. “Hopes are high that it will work, but even if this formulation does not immediately become a commercial product, we have learned to develop vaccines following the safety and quality requirements of regulatory agencies. This will enable us to create other vaccines for other diseases,” he notes.
SpiN-Tec is one of three vaccine candidates currently undergoing clinical trials in Brazil whose development has included some level of Brazilian involvement. The other two are RNA-MCTI-Cimatec-HDT and ButanVac. The former was developed by Seattle-based company HDT Biotech Corporation, with the participation of scientists from SENAI-CIMATEC, a teaching, research, and technological development center in Bahia. The latter uses a platform designed by teams from the Mount Sinai Health Network in New York and was developed by an international consortium that includes the Butantan Institute. Both have already completed phase 1 clinical trials and submitted the results to ANVISA, which on October 7 authorized the Cimatec-HDT formulation to proceed to the next phase. In October, the agency will analyze Butantan’s application for approval to conduct phase 2 trials of ButanVac.

CTVacina / UFMGA batch of UFMG’s SpiN-Tec vaccines destined for clinical trialsCTVacina / UFMG
The attempt to develop a vaccine entirely or partially in Brazil is part of a national effort to control all stages of the extensive and complex manufacturing chain of immunobiological products. Several research groups in the country carry out the initial stages of vaccine production, from formulating the active ingredient to conducting experiments on animals. This, however, is not enough to arrive at a product ready to be injected into people’s arms. Some of the links in the chain are missing. One is the absence of facilities with the capacity to increase vaccine production while following good manufacturing practices, which are needed to start clinical trials. “We do not have industrial development expertise in Brazil,” says Jorge Kalil, an immunologist from the Heart Institute (InCor) at the Hospital das Clínicas of the University of São Paulo (USP) School of Medicine who led the development of a nasal anti-COVID-19 formula that has already been tested on animals.
Since the beginning of 2020, the novel coronavirus has infected 620 million people worldwide and killed 6.5 million, according to figures from the World Health Organization (WHO). Experts have stated that the virus is here to stay, and after the initial phase during which the global population was completely unprotected, it is now likely to behave more like the flu virus, causing outbreaks in the winter months. This pattern will require the use of regular booster doses, which still need to be made more effective to generate more lasting protection.
Risk of dependency
It is for this reason that despite almost 50 vaccines already having been approved for human use worldwide, the WHO registered another 371 COVID-19 vaccine candidates under development in October (see chart). Experts in the field point out that the pandemic has revolutionized vaccine production technologies and countries not able to master them could become dependent on imports.
The three candidates undergoing human trials to which Brazil contributed all use different technologies to trigger the immune system.
Developed with funding from FAPESP, the Brazilian Ministry of Science, Technology, and Innovation (MCTI), and the Minas Gerais State Research Foundation (FAPEMIG), SpiN-Tec stimulates the production of antibodies against the SARS-CoV-2 spike protein, like most vaccines currently available on the market. Where it differs, however, is that as well as targeting the spike protein, which is on the surface of the virus exposed to attack by antibodies and undergoes frequent alterations, the team from Minas also added the nucleocapsid (N) protein—the envelope that protects the virus’s DNA and rarely changes from one strain to another. Synthesized by bacteria, the hybrid protein—known as SpiN—is then purified and mixed with an emulsion of oily compounds that encourage the activation of immune cells and amplify the immune response.
The vaccine’s efficacy was tested on two rodent species (mice and hamsters) at USP in Ribeirão Preto in collaboration with a team led by immunologist João Santana da Silva and virologist Luiz Tadeu Figueiredo. In all the animals, the SpiN protein–based vaccine triggered a powerful immune response. According to results published in the journal Nature Communications in August, SpiN-Tec stimulated both the activation of T lymphocytes, which directly combat the virus and infected cells, and B lymphocytes, which are responsible for producing antibodies. Administered in two doses, SpiN-Tec prevented illness in one type of rodent used as a model for mild disease and in another used to simulate severe disease, against the original SARS-CoV-2 virus and against the Delta and Omicron variants.
“The SpiN protein–based vaccine does not, by itself, induce the production of neutralizing antibodies,” explains Gazzinelli, who also works at the Oswaldo Cruz Foundation (FIOCRUZ) in Minas Gerais and is a visiting professor at USP in Ribeirão Preto, where he has received funding from FAPESP. “But as a booster dose, it stimulates the production of antibodies generated by previous vaccinations, as well as cellular immunity, providing double protection.”
The Cimatec-HDT vaccine, which is already being tested on human volunteers, uses RNA technology, but not the same technique adopted by the Pfizer-BioNTech and Moderna vaccines. Instead of using messenger RNA to carry the S protein recipe to the host’s cells, the Cimatec-HDT formulation uses self-replicating RNA, which multiplies inside cells several times before it starts spike protein production. The self-replicating RNA and lipid nanoparticles that surround it were developed in Seattle, with the participation of infectious disease specialist Roberto Badaró and biochemist Bruna Machado, both from SENAI-CIMATEC. “They have been collaborating with HDT on this platform to target diseases like Zika since before the pandemic,” says Valdir Gomes Barbosa, business manager at the Bahia-based research center. “In preclinical trials against the coronavirus carried out in the USA, the technology generated an immune response consistent with very low doses,” he reports. The technology has already been incorporated by SENAI-CIMATEC, which can now produce it on a laboratory scale.
In the first phase of the clinical trial, funded by the MCTI and conducted in Salvador at the beginning of the year, the vaccine was given to 90 adult volunteers who had previously been immunized against COVID-19 with other vaccines. They were divided into three groups that each received two doses of 1 microgram (µg), 5 µg, or 25 µg. According to Barbosa, the results, which have already been submitted to ANVISA, showed that the compound is safe and does not cause serious adverse effects, leading to approval of phase 2 trials. In this phase, scheduled to begin in the coming months, half of the 330 volunteers will receive two 5 µg or 10 µg doses—the dose size will be based on clinical trials already in progress in the USA, India, and South Korea. The other half will be given two doses of the Pfizer-BioNTech vaccine.

Nalini Vasconcelos / Senai-CimatecScientist performs a visual inspection of a vial of Cimatec-HDTNalini Vasconcelos / Senai-Cimatec
ButanVac, meanwhile, uses the Newcastle disease virus, which is practically harmless to humans, to introduce a more stable version of the S protein into the body (see Pesquisa FAPESP issue nos. 302 and 303). It was designed at the Icahn School of Medicine at Mount Sinai in New York with the aim of providing a cheap alternative that can be synthesized in flu vaccine factories. Authorized by ANVISA in July 2021, phase 1 trials evaluated the performance of the compound in 318 participants in Ribeirão Preto, in the interior of São Paulo State. The volunteers were separated into groups and given two doses of 1 µg, 3 µg, or 10 µg. “We observed a marked humoral response [production of antibodies], especially with the highest dose,” says Érique Miranda, clinical development manager of the Butantan Institute’s clinical trials division.
Two weeks after the second dose, ButanVac increased antibody production more than fourfold in 98.7% of participants. There were no serious adverse effects. The most common problems were pain on the injection site, reported in 89% of cases, headache (67%), fatigue (65%), body aches (58%), and fever (17%). “The results were very similar to the clinical trial of the same formulation carried out in Thailand,” says Miranda. The data from the Asian study were published as a preprint in September 2021 and suggest that the vaccine also triggers a cellular response. Once authorized, Butantan plans to start another trial with 400 volunteers from São Paulo and Rio de Janeiro.
The three candidates—SpiNTec, Cimatec-HDT, and ButanVac—are the survivors of a long obstacle course that leaves many competitors at the wayside. Federal and state research-funding agencies in Brazil began investing in the development of potential COVID-19 vaccines back in 2020. The MCTI funded the initial development of 16 compounds whose active ingredients were developed by scientists from Brazilian institutes or international partnerships that allowed the technology to be transferred to the country. “We are good at using foreign technology to produce vaccines in Brazil, but we have never made one ourselves from start to finish. We decided to encourage the development of a domestic capacity to produce immunizers,” says Marcelo Morales, Secretary of Scientific Research and Education at the MCTI, the department responsible for stimulating the development of COVID-19 vaccine candidates in the country.
Of the 16 compounds, five have completed animal testing and applied to share the R$115 million set aside for phase 1 and 2 trials, including the HDT-Cimatec and UFMG formulations. Two others are eligible to receive funding for clinical trials as soon as they receive approval from ANVISA for human testing: MultiCovax, created by Kalil’s team at InCor; and Versamune-CoV-2F, formulated by immunologist Célio Lopes Silva of USP in Ribeirão Preto, in partnership with São Paulo–based company Farmacore and American laboratory PDS Biotechnology. “In testing on animals, we showed that our compound generates high levels of antibodies and cellular response in the nasal mucosa,” says Kalil. His group now faces the challenge of obtaining an initial pilot batch to administer to human volunteers, which will have to be manufactured abroad at a cost of US$4 million due to the lack of options in Brazil. At the Federal University of Rio de Janeiro (UFRJ), a team led by biochemical engineer Leda Castilho is pushing to complete the animal testing phase of its UFRJVac vaccine later this year.
Project
Bivalent intranasal vaccine using influenza virus expressing the S protein (spike) of SARS-CoV-2: mechanisms of protection and lung injury (nº 20/05527-0); Grant Mechanism Regular Research Grant; Principal Investigator Ricardo Tostes Gazzinelli (USP); Investment R$283,052.33.
Scientific articles
CASTRO, J. T. et al. Promotion of neutralizing antibody-independent immunity to wild-type and SARS-CoV-2 variants of concern using an RBD-nucleocapsid fusion protein. Nature Communications. Aug. 17, 2022.
PITISUTTITHUM, P. et al. Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing Sars-CoV-2 spike: Interim results of a randomised, placebo-controlled, phase 1/2 trial. MedRxiv. Sept. 20, 2021.
Republish